Evidence for rate-dependent filtering of global extrinsic noise by biochemical reactions in mammalian cells
- PMID: 32407587
- PMCID: PMC7224485
- DOI: 10.15252/msb.20199335
Evidence for rate-dependent filtering of global extrinsic noise by biochemical reactions in mammalian cells
Abstract
Recent studies have revealed that global extrinsic noise arising from stochasticity in the intracellular biochemical environment plays a critical role in heterogeneous cell physiologies. However, it remains largely unclear how such extrinsic noise dynamically influences downstream reactions and whether it could be neutralized by cellular reactions. Here, using fluorescent protein (FP) maturation as a model biochemical reaction, we explored how cellular reactions might combat global extrinsic noise in mammalian cells. We developed a novel single-cell assay to systematically quantify the maturation rate and the associated noise for over a dozen FPs. By exploiting the variation in the maturation rate for different FPs, we inferred that global extrinsic noise could be temporally filtered by maturation reactions, and as a result, the noise levels for slow-maturing FPs are lower compared to fast-maturing FPs. This mechanism is validated by directly perturbing the maturation rates of specific FPs and measuring the resulting noise levels. Together, our results revealed a potentially general principle governing extrinsic noise propagation, where timescale separation allows cellular reactions to cope with dynamic global extrinsic noise.
Keywords: biological noise; chromophore maturation; fluorescent protein; global extrinsic noise.
© 2020 The Authors. Published under the terms of the CC BY 4.0 license.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
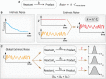
Schematic of an example first‐order reaction with a rate constant of k. The expression for the reaction rate is shown on the right, where C reactant denotes the cellular concentration of the reactant.
Schematic representations for intrinsic noise (left) and extrinsic noise (right). Intrinsic noise arises from the low copy number nature for some intracellular molecules. The schematic on the left shows the fluctuations of reactant concentration along an exponential decay curve. The schematic on the right illustrates the effect of extrinsic noise on the rate constant k, resulting in a dynamically fluctuating rate constant. Extrinsic noise can come from the fluctuations in the upstream components.
Global extrinsic noise can affect many biochemical reactions inside the cell. It has remained largely unclear how downstream reactions might combat with dynamic global extrinsic noise and whether the timescale of the reaction plays a role in affecting the cell‐to‐cell variability in the reaction.

Schematic of our single‐cell assay which decouples FP production and FP maturation into two separate signals in individual mammalian cells. The expression level of FP2 is measured by the nuclear fluorescence intensity signal of FP1, while the maturation of FP2 is measured by its own nuclear fluorescence intensity signal.
A representative filmstrip from the microscopy experiments characterizing mKate2 maturation. Fluorescence images for the H2B‐mTurquoise2 (for labeling the nucleus, top), Citrine‐SZ2 (the constitutive FP or FP1, middle), and mKate2‐2xNLS‐SZ1 (the inducible FP or FP2, bottom) are shown for indicated time points. The sale bar is 10 μm.
Single‐cell fluorescence intensity traces for quantifying mKate2 expression and mKate2 maturation. For these data, doxycycline was added at time zero. Traces in bold refer to the cell shown in (B). Traces for other cells (n = 30) are shown in light colors.
Boxplots showing the maturation times for 14 FPs measured by our assay. FPs are sorted by their spectra properties. Each box ranges from the first quartile to the third quartile of the data values, and the horizontal line inside indicates the median. The upper whisker is drawn up to the largest data value smaller than the third quartile plus 1.5× the interquartile range (IQR), and the lower whisker is drawn up to the lowest data value larger than the first quartile minus 1.5× IQR. The dataset for each FP contains n = 16–130 cells. Detailed information regarding the cell lines used can be found in Table EV1.

Construct designs and plasmid maps for the assay illustrated in Fig 2A. See Materials and Methods for more details regarding molecular cloning.
Overall procedures of the assay. Monoclonal or polyclonal cell lines were first plated on glass‐bottom 24‐well plates, which were then continuously imaged for certain periods on the microscope. The resulting images were processed to obtain single‐cell traces, which were then fitted to obtain maturation rates in individual cells. Example fitting results were shown in the fourth panel. The entire procedure was repeated for various FPs.
Experimental procedures and time‐course designs for measuring maturation kinetics (top) or for measuring the scaling ratios for fitting purposes (bottom). See Materials and Methods for more details.
Control experiment showing that the inducer doxycycline does not affect the production or localization of the constitutive FP (FP1). CHO cells were transiently transfected with plasmids containing the constitutively expressed mTurquoise2 (FP1) and the constitutively expressed nuclear labeling iRFP (iRFP‐H2B). Doxycycline was added in the middle of the experiment, and the resulting single‐cell trajectories were normalized by mean fluorescence. Error bars indicate ± SD (n = 74 cells).
Distributions of maturation times for 14 FPs. Data in Fig 2D were plotted as histograms. In order to compare between different FPs, each distribution was normalized by its mean value such that it is centered around one.

- A
Schematic diagram of a bidirectional promoter that drives the expression of two identical FPs but with different subcellular localizations (see Materials and Methods).
- B, C
Nuclear‐to‐cytoplasm ratio of mCherry (B) and mTurquoise2 (C) fluorescence signals acquired post‐induction at time zero. Error bars indicate ± SD. Datasets in (B) and (C) contain 245 and 135 cells, respectively.
- D
Analysis of extrinsic and intrinsic noise levels using the dataset in (B). Noise levels (η) were calculated at different time points with the formula described in Elowitz et al (2002).
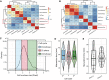
- A, B
Correlations of single‐cell FP maturation time with various cell state‐related factors for polyclonal CHO and U2OS cells (A) and for monoclonal CHO cells (B). In both scenarios, the cell state‐related parameters showed relatively low correlations with FP maturation kinetics. ParameterK: FP transcription rate multiplied by translation rate; ConsInitialValue: fluorescence value of the constitutively expressed FP in the first time point of fitting; InduInitialValue: fluorescence value of the inducible expressed FP in the first time point of fitting; CellType: different cell types used in our experiment (U2OS and CHO cells); CellArea: cell nuclear size in the first time point of fitting; Maturation Time: measured FP maturation time; EndFrame: the last time point of fitting; InitialFrame: the first time point of fitting. The dataset for (A) and (B) contains 150 and 73 cells, respectively.
- C, D
Maturation kinetics are not affected by cell cycle stages. (C) Initial nucleus size distribution for cells from cell cycle block‐and‐release experiments. Cells were then classified into three different cell cycle stages based on initial nucleus size (n = 151, 646, and 201 cells for G1, S, and G2 stage, respectively). (D) mCherry maturation kinetics is robust to cell cycle stage. Cells were classified into different cell cycle stages based on their initial nucleus sizes (n = 151, 646, and 201 cells for G1, S, and G2 stage, respectively). In the violin plot, each box ranges from the first quartile to the third quartile of the data values, and the horizontal line inside indicates the median. The upper whisker is drawn up to the largest data value smaller than the third quartile plus 1.5× the interquartile range (IQR), and the lower whisker is drawn up to the lowest data value larger than the first quartile minus 1.5× IQR. No significant difference was found among different groups (ANOVA test, df = 2, F = 0.179, P = 0.837).
- E
Comparison of FP maturation kinetics between two different CHO monoclones (n = 101 and 150 two monoclones, respectively). In the violin plot, the definitions of the box and whiskers are the same as in (D). No significant difference for mCherry maturation was found (t = −0.8168, df = 155.72, P = 0.4153).

Maturation time and the associated cell‐to‐cell variability for the 14 FPs. Data in Fig 2D were used to compute the coefficient of variation (i.e., the level of noise) for each set of single cells. The noise level shows a statistically significant negative correlation with the maturation time of the FP (Pearson's correlation coefficient: −0.80, t = −4.6614, df = 12, P < 0.001). The dataset for each FP contains n = 16–130 cells.
Scatter plot of the noise in FP maturation time versus the noise in FP production rate for the 14 FPs. The two noises showed an insignificant correlation (Pearson's correlation coefficient: 0.39, t = 1.48, df = 12, P = 0.16). The dataset for each FP contains n = 16–130 cells.
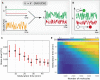
The rate constant of the maturation reaction is subjected to global extrinsic noise (schematic). In this phenomenological model, we assumed that global extrinsic noise arises from fluctuations in the cellular NAD(P)H level (see text for details). The resulting rate constant is stochastic and varies over time. Green line represents the temporally fluctuating rate constant for the FP with a faster maturation rate, while the red line is for the FP with a slower maturation rate.
Schematic illustrating the time‐averaging of global extrinsic noise. The timescales for the maturation reaction of two FP molecules are illustrated. As a FP molecule takes a longer time to mature (i.e., it has a lower rate constant), it would average the extrinsic noise over a larger time window.
Stochastic simulations of the phenomenological model. We incorporated the time‐averaging mechanism, and the results recapitulated a negative correlation between FP maturation noise and FP maturation time. Each condition contains 100 cells in the simulation, and each cell contains 2,000 molecules. Error bars indicate 95% confidence intervals of the mean by bootstrap.
Stochastic simulations of the model with varying numbers of FP molecules.

- A
Simulated results showing that the non‐genetic heterogeneity (coefficient of variation) in FP maturation time increases as the environmental noise level increases.
- B
Population‐averaged fluorescence intensities of the constitutive FP (top) and the inducible FP (bottom) in four different oxyrase treatment conditions (related to Fig 5B). Data from six different time points were shown, and error bars indicate ± SD. Multiple comparison test after Kruskal–Wallis test was performed on these data. NS means no significant difference. * means P < 0.05. These results suggest that while FP maturation was affected by oxyrase treatment, FP production was not affected.
- C
Scatter plot of the noise in FP maturation time and the noise in FP production rate for mCherry in oxygen limitation experiments (Fig 5B, Pearson's correlation coefficient: −0.21, t = −0.31, df = 2, P = 0.79). The dataset for each condition (from right to left) contains n = 143, 395, 299, and 529 cells, respectively. All error bars indicate 95% confidence intervals of the mean by bootstrap.
- D, E
Data from the maturation time measurement of mKate2 in oxygen limitation experiments (analogous to the experiments shown in Fig 5B). The dataset for each condition (from left to right) contains n = 207, 238, 193, and 157 cells, respectively. (D) Oxygen level limitation increased the maturation time of mKate2 and decreased the associated heterogeneity. (E) Scatter plot of the noise in FP maturation time and the noise in FP production rate for mKate2 in oxygen limitation experiments (Pearson's correlation coefficient: 0.15, t = 0.21, df = 2, P = 0.85). All error bars indicate 95% confidence intervals of the mean by bootstrap.

The maturation rate can be tuned by adjusting cellular oxygen level. The rate constant k′ is dependent on the oxygen level as shown by previous studies (Heim et al, 1994; Zhang et al, 2006; Iizuka et al, 2011).
Reducing the oxygen level in the media by oxygen‐scavenging enzyme reduces the maturation rate of mCherry, leading to lower noise levels. In this experiment, culture media were pre‐treated with a gradient of an oxygen‐scavenging enzyme and the corresponding maturation rates were measured (see Materials and Methods and also Fig EV4B–E). The dataset for each condition (from left to right) contains n = 143, 395, 299, and 529 cells, respectively.
The maturation rate for cofactor‐dependent FPs can be tuned by adjusting cofactor level. The rate constant k′ is dependent on the cofactor level as suggested by previous studies (Yu et al, 2015).
Near‐infrared FPs exhibited altered maturation rates and the associated noise levels in a biliverdin concentration‐dependent manner. Two monoclonal CHO cell lines (mIFP and iRFP) were used, and indicated concentration of biliverdin (or DMSO for 0 μM condition) was added to the culture medium (see also Fig EV5). The dataset for each condition (from left to right) contains n = 40, 39, 56, and 116 cells, respectively.

- A, B
Population‐averaged fluorescence intensities of the constitutive FP (top) and the inducible FP (bottom) under two different cofactor biliverdin concentrations (related to Fig 5D). Data for two separate near‐infrared FPs ((A) for iRFP and (B) for mIFP) at six different time points were shown, and error bars indicate ± SD. Multiple comparison test after Kruskal–Wallis test was performed on these data. NS means no significant difference. * means P < 0.05. These results suggest that while FP maturation was affected by biliverdin concentration, FP production was not affected.
- C
Scatter plot of the noise in FP maturation time and the noise in FP production rate for iRFP and mIFP under two different biliverdin conditions (Pearson's correlation coefficient: −0.3542, t = 0.54, df = 2, P = 0.65). The dataset contains n = 116, 39, 56, and 40 cells for mIFP_0, mIFP_10, iRFP_0, and iRFP_10, respectively. Error bars indicate 95% confidence intervals of the mean by bootstrap.
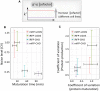
The natural variation in the cofactor biliverdin concentration in different cell lines allows us to study the cell line‐dependent maturation of near‐infrared FPs.
Cofactor‐dependent near‐infrared FPs exhibited altered maturation rates and the associated noise levels in a cell line‐dependent manner. Both iRFP (t = 3.8101, df = 27.024, P < 0.001) and mIFP (t = 11.576, df = 24.719, P < 0.001) showed significantly different maturation rates in CHO versus U2OS cells. The dataset for each condition (from left to right) contains n = 19, 42, 11, and 14 cells, respectively.
Scatter plot of the noise in FP maturation time and the noise in FP production rate for two cofactor‐dependent FPs (iRFP and mIFP) in two cell lines (CHO and U2OS) (Pearson's correlation coefficient: 0.80 t = 1.88, df = 2, P = 0.20). The dataset for each condition (from left to right) contains n = 14, 11, 19, and 42 cells, respectively.Data information: All error bars indicate 95% confidence intervals of the mean by bootstrap.
References
-
- Barkai N, Leibler S (1997) Robustness in simple biochemical networks. Nature 387: 913–917 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

