Evidence against Single-Electron Transfer in the Additions of Most Organomagnesium Reagents to Carbonyl Compounds
- PMID: 32407636
- PMCID: PMC7337984
- DOI: 10.1021/acs.joc.0c00481
Evidence against Single-Electron Transfer in the Additions of Most Organomagnesium Reagents to Carbonyl Compounds
Abstract
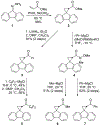
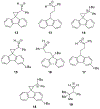
A radical clock system was developed to investigate single-electron transfer (SET) in the reactions of organomagnesium reagents with carbonyl compounds. The fluorenylcyclopropyl radical clock was selected because it is the fastest known radical clock. Additions of Grignard reagents to aldehydes or methyl ketones provided no evidence for ring-opened products that would indicate reaction through SET. Additions of some Grignard reagents to aromatic ketones, however, resulted in the formation of ring-opened products, suggesting SET.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Peltzer RM; Gauss J; Eisenstein O; Cascella M The Grignard Reaction – Unraveling a Chemical Puzzle. J. Am. Chem. Soc 2020, 142, 2984–2994. - PubMed
-
- Bartolo ND; Woerpel KA Mechanistic Insight into Additions of Allylic Grignard Reagents to Carbonyl Compounds. J. Org. Chem 2018, 83, 10197–10206. - PubMed
-
- Bartolo ND; Read JA; Valentín EM; Woerpel KA Reactions of Allylmagnesium Halides with Carbonyl Compounds: Reactivity, Structure, and Mechanism. Synthesis 2017, 49, 3237–3246.
-
- Otte DAL; Woerpel KA Evidence that Additions of Grignard Reagents to Aliphatic Aldehydes Do Not Involve Single-Electron-Transfer Processes. Org. Lett 2015, 17, 3906–3909. - PubMed
-
- Osztrovszky G; Holm T; Madsen R Ultrafast Grignard Addition Reactions in the Presence of Water. Org. Biomol. Chem 2010, 8, 3402–3404. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources