Uptake of methodological advances for synthesis of continuous and time-to-event outcomes would maximize use of the evidence base
- PMID: 32407766
- PMCID: PMC7435685
- DOI: 10.1016/j.jclinepi.2020.05.010
Uptake of methodological advances for synthesis of continuous and time-to-event outcomes would maximize use of the evidence base
Abstract
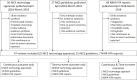
Objective: The objective of the study is to establish how often continuous and time-to-event outcomes are synthesized in health technology assessment (HTA), the statistical methods and software used in their analysis and how often evidence synthesis informs decision models.
Study design and setting: This is a review of National Institute of Health Research HTA reports, National Institute for Health and Care Excellence (NICE) technology appraisals, and NICE guidelines reporting quantitative meta-analysis or network meta-analysis of at least one continuous or time-to-event outcome published from April 01, 2018 to March 31, 2019.
Results: We identified 47 eligible articles. At least one continuous or time-to-event outcome was synthesized in 51% and 55% of articles, respectively. Evidence synthesis results informed decision models in two-thirds of articles. The review and expert knowledge identified five areas where methodology is available for improving the synthesis of continuous and time-to-event outcomes: i) outcomes reported on multiple scales, ii) reporting of multiple related outcomes, iii) appropriateness of the additive scale, iv) reporting of multiple time points, and v) nonproportional hazards. We identified three anticipated barriers to the uptake and implementation of these methods: i) statistical expertise, ii) software, and iii) reporting of trials.
Conclusion: Continuous and time-to-event outcomes are routinely reported in HTA. However, increased uptake of methodological advances could maximize the evidence base used to inform the decision making process.
Keywords: Clinical decision-making; Continuous outcomes; Decision models; Evidence synthesis; Health technology assessment; Time-to-event outcomes.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Modelling approaches for histology-independent cancer drugs to inform NICE appraisals: a systematic review and decision-framework.Health Technol Assess. 2021 Dec;25(76):1-228. doi: 10.3310/hta25760. Health Technol Assess. 2021. PMID: 34990339
-
Appraisals by Health Technology Assessment Agencies of Economic Evaluations Submitted as Part of Reimbursement Dossiers for Oncology Treatments: Evidence from Canada, the UK, and Australia.Curr Oncol. 2022 Oct 13;29(10):7624-7636. doi: 10.3390/curroncol29100602. Curr Oncol. 2022. PMID: 36290879 Free PMC article.
-
Avoiding and identifying errors in health technology assessment models: qualitative study and methodological review.Health Technol Assess. 2010 May;14(25):iii-iv, ix-xii, 1-107. doi: 10.3310/hta14250. Health Technol Assess. 2010. PMID: 20501062 Review.
-
Measuring Survival Benefit in Health Technology Assessment in the Presence of Nonproportional Hazards.Value Health. 2019 Apr;22(4):431-438. doi: 10.1016/j.jval.2019.01.005. Epub 2019 Mar 23. Value Health. 2019. PMID: 30975394
Cited by
-
Safety, efficacy, and cost-effectiveness evaluation of systemic treatments for refractory colorectal cancer: a systematic review and modeling study.Health Econ Rev. 2025 Apr 11;15(1):33. doi: 10.1186/s13561-025-00622-x. Health Econ Rev. 2025. PMID: 40214895 Free PMC article.
-
Weekly versus tri-weekly paclitaxel with carboplatin for first-line treatment in women with epithelial ovarian cancer.Cochrane Database Syst Rev. 2022 Feb 21;2(2):CD012007. doi: 10.1002/14651858.CD012007.pub2. Cochrane Database Syst Rev. 2022. PMID: 35188221 Free PMC article.
-
Challenges of modelling approaches for network meta-analysis of time-to-event outcomes in the presence of non-proportional hazards to aid decision making: Application to a melanoma network.Stat Methods Med Res. 2022 May;31(5):839-861. doi: 10.1177/09622802211070253. Epub 2022 Jan 19. Stat Methods Med Res. 2022. PMID: 35044255 Free PMC article.
-
Challenges in conducting fractional polynomial and standard parametric network meta-analyses of immune checkpoint inhibitors for first-line advanced renal cell carcinoma.J Comp Eff Res. 2023 Aug;12(8):e230004. doi: 10.57264/cer-2023-0004. Epub 2023 Jul 11. J Comp Eff Res. 2023. PMID: 37431849 Free PMC article. Review.
-
Coronary Artery Bypass Grafting Versus Percutaneous Coronary Intervention for Multivessel Coronary Artery Disease: A One-Stage Meta-Analysis.Front Cardiovasc Med. 2022 Mar 25;9:822228. doi: 10.3389/fcvm.2022.822228. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35402572 Free PMC article.
References
-
- Cooper N., Sutton A., Achana F., Welton N. Canadian Agency for Drugs and Technologies in Health; Ottawa: 2016. Use of network meta-analysis to inform clinical parameters in economic evaluations.
-
- Lumley T. Network meta-analysis for indirect treatment comparisons. Stat Med. 2002;21:2313–2324. - PubMed
-
- Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods. 2012;3(2):80–97. - PubMed
-
- National Institute of Health and Care Excellence Guide to the methods of technology appraisal 2004. 2004. https://webarchive.nationalarchives.gov.uk/20080611223138/http://www.nic... Available at. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous