An exploratory analysis on the influence of genetic variants on weight gain among etonogestrel contraceptive implant users
- PMID: 32407811
- PMCID: PMC7483263
- DOI: 10.1016/j.contraception.2020.05.002
An exploratory analysis on the influence of genetic variants on weight gain among etonogestrel contraceptive implant users
Abstract
Objective: To identify genetic variants associated with weight gain related to etonogestrel contraceptive implant use.
Study design: We conducted a retrospective analysis from a parent pharmacogenomic study of healthy, reproductive-aged women using etonogestrel implants. We reviewed medical records to calculate objective weight changes from implant insertion to study enrollment and asked participants about subjective weight gain (yes/no) during contraceptive implant use. We used genotyping data (99 genetic variants) from the parent study to conduct backward-stepwise generalized linear modeling to identify genetic variants associated with objective weight changes.
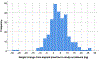
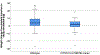
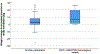
Results: Among 276 ethnically diverse participants, median body-mass index (BMI) was 25.8 kg/m2 (range 18.5-48.1). We found a median weight change of +3.2 kg (range -27.6 to +26.5) from implant insertion to study enrollment. Report of subjective weight gain had minimal agreement with measured weight gain during implant use (Cohen's kappa = 0.21). Our final generalized linear model contained two variables associated with objective weight change that met conservative statistical significance (p < 5.0 × 10-4). Participants with two copies (homozygous) of the ESR1 rs9340799 variant on average gained 14.1 kg more than all other participants (p = 1.4 × 10-4). Higher enrollment BMI was also associated with objective weight gain (β = 0.54, p = 9.4 × 10-12).
Conclusion: Genetic variants in the estrogen receptor 1 (ESR1) do not have known associations with obesity or metabolic syndrome, but there is physiologic plausibility for a progestin-mediated genetic association between ESR1 and weight gain. Additional genetic research is needed to substantiate our findings and elucidate further advances in individualized counseling on the risk of weight gain with exogenous steroid hormones.
Implications: Genetic variation in the estrogen receptor 1 gene may account for variability in weight gain among etonogestrel contraceptive implant users. If these findings can be replicated with other progestin-containing medications, we may be able to better individualize contraceptive counseling.
Keywords: Contraception; Estrogen receptor; Etonogestrel; Obesity; Pharmacogenomics; Weight gain.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
Similar articles
-
Applicability of ancestral genotyping in pharmacogenomic research with hormonal contraception.Clin Transl Sci. 2021 Sep;14(5):1713-1718. doi: 10.1111/cts.13014. Epub 2021 May 2. Clin Transl Sci. 2021. PMID: 33650294 Free PMC article.
-
Influence of Genetic Variants on Steady-State Etonogestrel Concentrations Among Contraceptive Implant Users.Obstet Gynecol. 2019 Apr;133(4):783-794. doi: 10.1097/AOG.0000000000003189. Obstet Gynecol. 2019. PMID: 30870275 Free PMC article.
-
Relationship between patient characteristics and serum etonogestrel concentrations in contraceptive implant users.Contraception. 2019 Jul;100(1):37-41. doi: 10.1016/j.contraception.2019.03.045. Epub 2019 Apr 10. Contraception. 2019. PMID: 30980827 Free PMC article.
-
Association of Etonogestrel-Releasing Contraceptive Implant with Reduced Weight Gain in an Exclusively Breastfed Infant: Report and Literature Review.Breastfeed Med. 2016 May;11(4):203-6. doi: 10.1089/bfm.2016.0017. Epub 2016 Mar 31. Breastfeed Med. 2016. PMID: 27032034 Free PMC article. Review.
-
Best Practices for Counseling Adolescents about the Etonogestrel Implant.J Pediatr Adolesc Gynecol. 2020 Oct;33(5):448-454. doi: 10.1016/j.jpag.2020.06.022. Epub 2020 Jul 2. J Pediatr Adolesc Gynecol. 2020. PMID: 32621879 Review.
Cited by
-
Early removal of the etonogestrel contraceptive implant in Spanish women: a prospective cohort study.Front Med (Lausanne). 2024 Jan 23;11:1172793. doi: 10.3389/fmed.2024.1172793. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38323032 Free PMC article.
-
Applicability of ancestral genotyping in pharmacogenomic research with hormonal contraception.Clin Transl Sci. 2021 Sep;14(5):1713-1718. doi: 10.1111/cts.13014. Epub 2021 May 2. Clin Transl Sci. 2021. PMID: 33650294 Free PMC article.
-
An exploratory study on the possible association of serum etonogestrel concentrations with mood concerns and symptoms among contraceptive implant users.Contraception. 2024 Jan;129:110298. doi: 10.1016/j.contraception.2023.110298. Epub 2023 Oct 4. Contraception. 2024. PMID: 37802462 Free PMC article.
References
-
- Croxatto HB, Urbancsek J, Massai R, Bennink HC, van Beek A. A multicentre efficacy and safety study of the single contraceptive implant Implanon®. Hum Reprod. 1999;14:976–81. - PubMed
-
- Bahamondes L, Brache V, Ali M, Habib N. A multicenter randomized clinical trial of etonogestrel and levonorgestrel contraceptive implants with nonrandomized copper intrauterine device controls: effect on weight variations up to 3 years after placement. Contraception. 2018;98:181–7. - PubMed
-
- U.S. Food and Drug Administration. Drug Development and Drug Interactions: Table of Substrates, Inhibitors, and Inducers, https://www.fda.gov/drugs/drug-interactions-labeling/drug-development-an...; 2020. [accessed 15 March 2020]
-
- Modesto W, Dal Ava N, Monteiro I, Bahamondes L. Body composition and bone mineral density in users of the etonogestrel-releasing contraceptive implant. Arch Gynecol Obstet. 2015;292:1387–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

