Data set for the reporting of pheochromocytoma and paraganglioma: explanations and recommendations of the guidelines from the International Collaboration on Cancer Reporting
- PMID: 32407815
- PMCID: PMC7655677
- DOI: 10.1016/j.humpath.2020.04.012
Data set for the reporting of pheochromocytoma and paraganglioma: explanations and recommendations of the guidelines from the International Collaboration on Cancer Reporting
Abstract
Background and objectives: The International Collaboration on Cancer Reporting (ICCR) is a not-for-profit to develop evidence-based, internationally agreed-upon standardized data sets for each anatomic site, to be used throughout the world. Providing global standardization of pathology tumor classification, staging, and other reporting elements will lead to improved patient management and enhanced epidemiological research.
Methods: Pheochromocytoma and paraganglioma are uncommon and are frequently overlooked in registry data sets. Malignant criteria have previously been defined only when there was metastatic disease.
Results: With recent recognition of a significant inheritance association and the development of risk stratification tools, this data set was created in order to obtain more meaningful outcomes and management data, using similar criteria across the global pathology community. Issues related to key core and non-core elements, especially clinical hormonal status, familial history, tumor focality, proliferative fraction, adverse or risk stratification features, and ancillary techniques, are discussed in the context of daily application to these types of specimens.
Conclusions: The ICCR data set, developed by an international panel of endocrine organ specialists, establishes a pathology-standardized reporting guide for pheochromocytoma and paraganglioma.
Keywords: Checklist; Data set; ICCR; Paraganglioma; Pheochromocytoma; Structured report; Synoptic reporting.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures
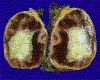



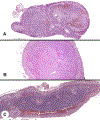
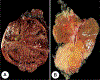

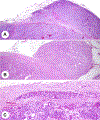



References
-
- Srigley J, Lankshear S, Brierley J, McGowan T, Divaris D, Yurcan M, et al. Closing the quality loop: facilitating improvement in oncology practice through timely access to clinical performance indicators. J Oncol Pract 2013;9:e255–61. - PubMed
-
- Ellis DW, Srigley J. Does standardised structured reporting contribute to quality in diagnostic pathology? The importance of evidence-based datasets. Virchows Arch 2016;468:51–9. - PubMed
-
- Tischler AS, Asa SL, Clifton-Bligh R, de Krijger RR, Kimura N, Komminoth P, et al. Phaeochromocytoma and Paraganglioma Histopathology Reporting Guide. Sydney, Australia: International Collaboration on Cancer Reporting; 2019.
-
- Giordano T, Berney D, de Krijger R, Erickson LA, Fassnacht M, Mete O, et al. Carcinoma of the Adrenal Cortex Histopathology Reporting Guide. Sydney, Australia: International Collaboration on Cancer Reporting; 2019.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

