QT interval prolongation and torsade de pointes in patients with COVID-19 treated with hydroxychloroquine/azithromycin
- PMID: 32407884
- PMCID: PMC7214283
- DOI: 10.1016/j.hrthm.2020.05.014
QT interval prolongation and torsade de pointes in patients with COVID-19 treated with hydroxychloroquine/azithromycin
Abstract
Background: There is no known effective therapy for patients with coronavirus disease 2019 (COVID-19). Initial reports suggesting the potential benefit of hydroxychloroquine/azithromycin (HY/AZ) have resulted in massive adoption of this combination worldwide. However, while the true efficacy of this regimen is unknown, initial reports have raised concerns about the potential risk of QT interval prolongation and induction of torsade de pointes (TdP).
Objective: The purpose of this study was to assess the change in corrected QT (QTc) interval and arrhythmic events in patients with COVID-19 treated with HY/AZ.
Methods: This is a retrospective study of 251 patients from 2 centers who were diagnosed with COVID-19 and treated with HY/AZ. We reviewed electrocardiographic tracings from baseline and until 3 days after the completion of therapy to determine the progression of QTc interval and the incidence of arrhythmia and mortality.
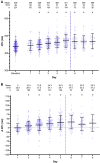
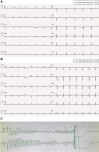
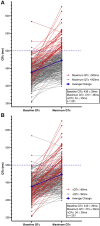
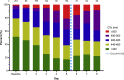
Results: The QTc interval prolonged in parallel with increasing drug exposure and incompletely shortened after its completion. Extreme new QTc interval prolongation to >500 ms, a known marker of high risk of TdP, had developed in 23% of patients. One patient developed polymorphic ventricular tachycardia suspected as TdP, requiring emergent cardioversion. Seven patients required premature termination of therapy. The baseline QTc interval of patients exhibiting extreme QTc interval prolongation was normal.
Conclusion: The combination of HY/AZ significantly prolongs the QTc interval in patients with COVID-19. This prolongation may be responsible for life-threatening arrhythmia in the form of TdP. This risk mandates careful consideration of HY/AZ therapy in light of its unproven efficacy. Strict QTc interval monitoring should be performed if the regimen is given.
Keywords: Azithromycin; COVID-19; Hydroxychloroquine; QT interval; Torsade de pointes.
© 2020 Heart Rhythm Society. All rights reserved.
Figures
References
-
- Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [published online ahead of print March 9, 2020]. Clin Infect Dis. 10.1093/cid/ciaa237. - DOI - PMC - PubMed
-
- Ferner R.E., Aronson J.K. Chloroquine and hydroxychloroquine in covid-19. BMJ. 2020;369:m1432. - PubMed
-
- Chen C.Y., Wang F.L., Lin C.C. Chronic hydroxychloroquine use associated with QT prolongation and refractory ventricular arrhythmia. Clin Toxicol (Phila) 2006;44:173–175. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

