Compassionate remdesivir treatment of severe Covid-19 pneumonia in intensive care unit (ICU) and Non-ICU patients: Clinical outcome and differences in post-treatment hospitalisation status
- PMID: 32407959
- PMCID: PMC7212963
- DOI: 10.1016/j.phrs.2020.104899
Compassionate remdesivir treatment of severe Covid-19 pneumonia in intensive care unit (ICU) and Non-ICU patients: Clinical outcome and differences in post-treatment hospitalisation status
Abstract
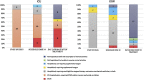
SARS-CoV-2 is causing an increasing number of deaths worldwide because no effective treatment is currently available. Remdesivir has shown in vitro activity against coronaviruses and is a possible antiviral treatment for SARS-CoV-2 infection. This prospective (compassionate), open-label study of remdesivir, which was conducted at Luigi Sacco Hospital, Milan, Italy, between February 23 and March 20, 2020, involved patients with SARS-CoV-2 pneumonia aged ≥18 years undergoing mechanical ventilation or with an oxygen saturation level of ≤94 % in air or a National Early Warning Score 2 of ≥4. The primary outcome was the change in clinical status based on a 7-category ordinal scale (1 = not hospitalised, resuming normal daily activities; 7 = deceased). The 35 patients enrolled from February 23 to March 20, 2020, included 18 in intensive care unit (ICU), and 17 in our infectious diseases ward (IDW). The 10-day course of remdesivir was completed by 22 patients (63 %) and discontinued by 13, of whom eight (22.8 %) discontinued because of adverse events. The median follow-up was 39 days (IQR 25-44). At day 28, 14 (82.3 %) patients from IDW were discharged, two were still hospitalized and one died (5.9 %), whereas in ICU 6 (33.3 %) were discharged, 8 (44.4 %) patients died, three (16.7 %) were still mechanically ventilated and one (5.6 %) was improved but still hospitalized. Hypertransaminasemia and acute kidney injury were the most frequent severe adverse events observed (42.8 % and 22.8 % of the cases, respectively). Our data suggest that remdesivir can benefit patients with SARS-CoV-2 pneumonia hospitalised outside ICU where clinical outcome was better and adverse events are less frequently observed. Ongoing randomised controlled trials will clarify its real efficacy and safety, who to treat, and when.
Keywords: COVID-19; Remdesivir; SARS-CoV-2.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures
References
-
- Coronavirus: la situazione dei contagi in Italia, 29 aprile 2020. http://www.protezionecivile.gov.it/media-comunicazione/comunicati-stampa....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous