Dolutegravir plus lamivudine for maintenance of HIV viral suppression in adults with and without historical resistance to lamivudine: 48-week results of a non-randomized, pilot clinical trial (ART-PRO)
- PMID: 32408111
- PMCID: PMC7225620
- DOI: 10.1016/j.ebiom.2020.102779
Dolutegravir plus lamivudine for maintenance of HIV viral suppression in adults with and without historical resistance to lamivudine: 48-week results of a non-randomized, pilot clinical trial (ART-PRO)
Erratum in
-
Corrigendum to "Dolutegravir plus lamivudine for maintenance of HIV viral suppression in adults with and without historical resistance to lamivudine: 48-week results of a non-randomized, pilot clinical trial (ART-PRO)".EBioMedicine. 2023 Mar;89:104486. doi: 10.1016/j.ebiom.2023.104486. Epub 2023 Feb 18. EBioMedicine. 2023. PMID: 36806001 Free PMC article. No abstract available.
Abstract
Background: We investigated the efficacy of a switch to dolutegravir plus lamivudine in aviremic individuals without evidence of persistent lamivudine resistance-associated mutations in baseline proviral DNA population sequencing.
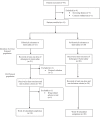
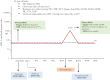
Methods: Open-label, single-arm, 48-week pilot trial. HIV-1 infected adults, naïve to integrase inhibitors, with CD4+ above 350 cell/μL and fewer than 50 HIV-1 RNA copies per mL the year prior to study entry switched to dolutegravir plus lamivudine. Participants were excluded if baseline proviral DNA population genotyping detected lamivudine resistance-associated mutations. To detect resistance minority variants, proviral DNA next-generation sequencing was retrospectively performed from baseline samples. Primary efficacy endpoint was proportion of participants with fewer than 50 HIV-1 RNA copies per mL at week 48. Safety and tolerability outcomes were incidence of adverse events and treatment discontinuations. ART-PRO is registered with ClinicalTrials.gov, NCT03539224.
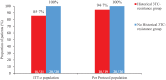
Findings: 41 participants switched to dolutegravir plus lamivudine, 21 with lamivudine resistance mutations in historical plasma genotypes. Baseline next-generation sequencing detected lamivudine resistance mutations (M184V/I and/or K65R/E/N) over a 5% threshold in 15/21 (71·4%) and 3/20 (15%) of participants with and without history of lamivudine resistance, respectively. At week 48, 92·7% of participants (38/41) had fewer than 50 HIV-1 RNA copies per mL. There were no cases of virologic failure. Three participants with historical lamivudine resistance were prematurely discontinued from the study (2 protocol violations, one adverse event). Ten participants (4 in the group with historical lamivudine resistance) had a transient viral rebound, all resuppressed on dolutegravir plus lamivudine. There were 28 drug-related adverse events, only one leading to discontinuation.
Interpretation: In this pilot trial, dolutegravir plus lamivudine was effective in maintaining virologic control despite past historical lamivudine resistance and presence of archived lamivudine resistance-associated mutations detected by next generation sequencing. Further studies are needed to confirm our results.
Funding: Fondo de Investigaciones Sanitarias, Instituto de Salud Carlos III PI16/00837-PI16/00678.
Copyright © 2020 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest RDM reports grants from Fondo de Investigaciones Sanitarias, during the conduct of the study; personal fees and non-financial support from Janssen, non-financial support from ViiV, non-financial support from Gilead, outside the submitted work. DR reports personal fees from Gilead Sciences Inc, personal fees from Janssen Cilag, grants and personal fees from ViiV Healthcare, outside the submitted work. LD reports payment for lectures from Gilead and Janssen, and financial support for expert courses and congress from Merck Sharp and Dome, Gilead and Abbvie, outside the submitted work. RM reports grants from Juan Rodes 18/00039, during the conduct of the study; personal fees from ViiV Health care, personal fees from Janssen Cilag, outside the submitted work. OB reports non-financial support from GILEAD, personal fees from GILEAD, personal fees from VIIV, grants from ViiV, non-financial support from MSD, grants from VIIV, outside the submitted work. AE reports grants from Instituto de Salud Carlos III, during the conduct of the study. PA reports personal fees from ViiV Healthcare, outside the submitted work. NS reports personal fees from Janssen, personal fees from Gilead, outside the submitted work. LB has nothing to disclose. MG reports grants and personal fees from Hologic, grants from Roche diagnostics, grants from Beckman coulter, personal fees from ViiV Health Care, grants from Instituto de Salud Carlos III, outside the submitted work. JC reports grants from Instituto de Salud Carlos III - Ministerio de Ciencia, Innovación y Universidades, outside the submitted work. MS reports personal fees from Janssen Cilag, personal fees from ViiV Healthcare, outside the submitted work. BA has nothing to disclose. AH has nothing to disclose. MM has nothing to disclose. JC has nothing to disclose. VM reports personal fees from ViiV Health Care, personal fees from Gilead Sciences, personal fees and non-financial support from Janssen Cilag, personal fees from Merck Sharp & Dohme, outside the submitted work. LM reports personal fees from Gilead, personal fees from Viiv, personal fees from MSD, personal fees from Janssen, outside the submitted work. RR reports personal fees from ViiV Healht Care, personal fees from Gilead Sciences, personal fees from Janssen Cilag, personal fees from Merck Sharp & Dohme, outside the submitted work. RD has received conference fees from ViiV. FP reports grants from Instituto de Salud Carlos III, during the conduct of the study; personal fees from Gilead Sciences, personal fees from Janssen, personal fees from MSD, personal fees from ViiV Healthcare, outside the submitted work. JRA reports grants from Instituto de Salud Carlos III, during the conduct of the study; grants and personal fees from VIIV, Gilead, personal fees from Janssen, MSD, Alexa, TEVA, outside the submitted work.
Figures
Comment in
-
Dolutegravir plus lamivudine for hiv treatment: Does the historical genotype really matter?EBioMedicine. 2020 Jun;56:102820. doi: 10.1016/j.ebiom.2020.102820. Epub 2020 Jun 5. EBioMedicine. 2020. PMID: 32512519 Free PMC article. No abstract available.
References
-
- EACS Guidelines version 10.0, November 2019. 2019;(November).
-
- Panel on Antiretroviral Guidelines for Adults and Adolescents Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Dep Heal Hum Serv. 2018
-
- van Wyk J, Ajana F, Bisshop F, De Wit S, Osiyemi O, Portilla J. Efficacy and Safety of Switching to Dolutegravir/Lamivudine Fixed-Dose Two-Drug Regimen Versus Continuing a Tenofovir Alafenamide–Based Three- or Four-Drug Regimen for Maintenance of Virologic Suppression in Adults With HIV-1: Phase 3, Randomized. Non-inf. Clin Infect Dis. 2020 Jan - PMC - PubMed
-
- Charpentier C, Peytavin G, Burdet C, Landman R, Lê M, Katlama C. Residual HIV-1 RNA, HIV-1 DNA, and Drug Plasma Cmin in Dual DTG+3TC. ANRS 167 Lamidol. CROI 2019. 2019
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

