Neurofilaments: neurobiological foundations for biomarker applications
- PMID: 32408345
- PMCID: PMC7363489
- DOI: 10.1093/brain/awaa098
Neurofilaments: neurobiological foundations for biomarker applications
Abstract
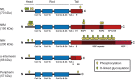
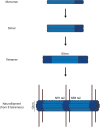
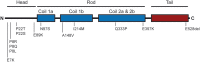
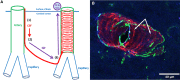
Interest in neurofilaments has risen sharply in recent years with recognition of their potential as biomarkers of brain injury or neurodegeneration in CSF and blood. This is in the context of a growing appreciation for the complexity of the neurobiology of neurofilaments, new recognition of specialized roles for neurofilaments in synapses and a developing understanding of mechanisms responsible for their turnover. Here we will review the neurobiology of neurofilament proteins, describing current understanding of their structure and function, including recently discovered evidence for their roles in synapses. We will explore emerging understanding of the mechanisms of neurofilament degradation and clearance and review new methods for future elucidation of the kinetics of their turnover in humans. Primary roles of neurofilaments in the pathogenesis of human diseases will be described. With this background, we then will review critically evidence supporting use of neurofilament concentration measures as biomarkers of neuronal injury or degeneration. Finally, we will reflect on major challenges for studies of the neurobiology of intermediate filaments with specific attention to identifying what needs to be learned for more precise use and confident interpretation of neurofilament measures as biomarkers of neurodegeneration.
Keywords: biomarkers; neurodegeneration; neurofilaments; neuroinflammation; traumatic brain injury.
© The Author(s) (2020). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures

References
-
- Abe A, Numakura C, Saito K, Koide H, Oka N, Honma A, et al. Neurofilament light chain polypeptide gene mutations in Charcot-Marie-Tooth disease: nonsense mutation probably causes a recessive phenotype. J Hum Genet 2009; 54: 94–7. - PubMed

