Usutu Virus Infection of Embryonated Chicken Eggs and a Chicken Embryo-Derived Primary Cell Line
- PMID: 32408481
- PMCID: PMC7291025
- DOI: 10.3390/v12050531
Usutu Virus Infection of Embryonated Chicken Eggs and a Chicken Embryo-Derived Primary Cell Line
Abstract
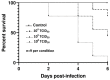
Usutu virus (USUV) is a mosquito-borne flavivirus, closely related to the West Nile virus (WNV). Similar to WNV, USUV may cause infections in humans, with occasional, but sometimes severe, neurological complications. Further, USUV can be highly pathogenic in wild and captive birds and its circulation in Europe has given rise to substantial avian death. Adequate study models of this virus are still lacking but are critically needed to understand its pathogenesis and virulence spectrum. The chicken embryo is a low-cost, easy-to-manipulate and ethically acceptable model that closely reflects mammalian fetal development and allows immune response investigations, drug screening, and high-throughput virus production for vaccine development. While former studies suggested that this model was refractory to USUV infection, we unexpectedly found that high doses of four phylogenetically distinct USUV strains caused embryonic lethality. By employing immunohistochemistry and quantitative reverse transcriptase-polymerase chain reaction, we demonstrated that USUV was widely distributed in embryonic tissues, including the brain, retina, and feather follicles. We then successfully developed a primary cell line from the chorioallantoic membrane that was permissive to the virus without the need for viral adaptation. We believe the future use of these models would foster a significant understanding of USUV-induced neuropathogenesis and immune response and allow the future development of drugs and vaccines against USUV.
Keywords: Usutu virus; chicken embryo; chorioallantoic membrane; flavivirus; model; primary culture; replication.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










Similar articles
-
In Vitro and In Vivo Models to Study the Zoonotic Mosquito-Borne Usutu Virus.Viruses. 2020 Sep 30;12(10):1116. doi: 10.3390/v12101116. Viruses. 2020. PMID: 33008141 Free PMC article. Review.
-
Pathogenesis and shedding of Usutu virus in juvenile chickens.Emerg Microbes Infect. 2021 Dec;10(1):725-738. doi: 10.1080/22221751.2021.1908850. Emerg Microbes Infect. 2021. PMID: 33769213 Free PMC article.
-
Limited pathogenicity of Usutu virus for the domestic chicken (Gallus domesticus).Avian Pathol. 2005 Oct;34(5):392-5. doi: 10.1080/03079450500268500. Avian Pathol. 2005. PMID: 16236570
-
Effect of blood source on vector competence of Culex pipiens biotypes for Usutu virus.Parasit Vectors. 2021 Apr 8;14(1):194. doi: 10.1186/s13071-021-04686-6. Parasit Vectors. 2021. PMID: 33832527 Free PMC article.
-
An overview of Usutu virus.Microbes Infect. 2017 Jul-Aug;19(7-8):382-387. doi: 10.1016/j.micinf.2017.05.003. Epub 2017 Jun 8. Microbes Infect. 2017. PMID: 28602915 Review.
Cited by
-
In Vitro and In Vivo Models to Study the Zoonotic Mosquito-Borne Usutu Virus.Viruses. 2020 Sep 30;12(10):1116. doi: 10.3390/v12101116. Viruses. 2020. PMID: 33008141 Free PMC article. Review.
-
Differential neurovirulence of Usutu virus lineages in mice and neuronal cells.J Neuroinflammation. 2021 Jan 6;18(1):11. doi: 10.1186/s12974-020-02060-4. J Neuroinflammation. 2021. PMID: 33407600 Free PMC article.
-
Human Usutu Virus Infections in Europe: A New Risk on Horizon?Viruses. 2022 Dec 27;15(1):77. doi: 10.3390/v15010077. Viruses. 2022. PMID: 36680117 Free PMC article. Review.
-
Belgian Culex pipiens pipiens are competent vectors for West Nile virus while Culex modestus are competent vectors for Usutu virus.PLoS Negl Trop Dis. 2023 Sep 20;17(9):e0011649. doi: 10.1371/journal.pntd.0011649. eCollection 2023 Sep. PLoS Negl Trop Dis. 2023. PMID: 37729233 Free PMC article.
-
Relevance of oxidative stress in inhibition of eIF2 alpha phosphorylation and stress granules formation during Usutu virus infection.PLoS Negl Trop Dis. 2021 Jan 25;15(1):e0009072. doi: 10.1371/journal.pntd.0009072. eCollection 2021 Jan. PLoS Negl Trop Dis. 2021. PMID: 33493202 Free PMC article.
References
-
- IZSAM CESME Integrated Surveillance of West Nile and Usutu Virus. [(accessed on 2 December 2019)]; Available online: https://westnile.izs.it/j6_wnd/home.
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources

