Reactive Oxygen Species, Metabolic Plasticity, and Drug Resistance in Cancer
- PMID: 32408513
- PMCID: PMC7279373
- DOI: 10.3390/ijms21103412
Reactive Oxygen Species, Metabolic Plasticity, and Drug Resistance in Cancer
Abstract
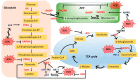
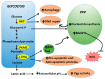
The metabolic abnormality observed in tumors is characterized by the dependence of cancer cells on glycolysis for their energy requirements. Cancer cells also exhibit a high level of reactive oxygen species (ROS), largely due to the alteration of cellular bioenergetics. A highly coordinated interplay between tumor energetics and ROS generates a powerful phenotype that provides the tumor cells with proliferative, antiapoptotic, and overall aggressive characteristics. In this review article, we summarize the literature on how ROS impacts energy metabolism by regulating key metabolic enzymes and how metabolic pathways e.g., glycolysis, PPP, and the TCA cycle reciprocally affect the generation and maintenance of ROS homeostasis. Lastly, we discuss how metabolic adaptation in cancer influences the tumor's response to chemotherapeutic drugs. Though attempts of targeting tumor energetics have shown promising preclinical outcomes, the clinical benefits are yet to be fully achieved. A better understanding of the interaction between metabolic abnormalities and involvement of ROS under the chemo-induced stress will help develop new strategies and personalized approaches to improve the therapeutic efficiency in cancer patients.
Keywords: cancer; drug resistance; metabolic adaptation; reactive oxygen species.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures
Similar articles
-
Cancer cells change their glucose metabolism to overcome increased ROS: One step from cancer cell to cancer stem cell?Biomed Pharmacother. 2019 Apr;112:108690. doi: 10.1016/j.biopha.2019.108690. Epub 2019 Feb 22. Biomed Pharmacother. 2019. PMID: 30798124 Review.
-
Cisplatin Resistance and Redox-Metabolic Vulnerability: A Second Alteration.Int J Mol Sci. 2021 Jul 9;22(14):7379. doi: 10.3390/ijms22147379. Int J Mol Sci. 2021. PMID: 34298999 Free PMC article. Review.
-
Drug discovery strategies in the field of tumor energy metabolism: Limitations by metabolic flexibility and metabolic resistance to chemotherapy.Biochim Biophys Acta Bioenerg. 2017 Aug;1858(8):674-685. doi: 10.1016/j.bbabio.2017.02.005. Epub 2017 Feb 16. Biochim Biophys Acta Bioenerg. 2017. PMID: 28213330 Review.
-
Recent advances in the role of AMP-activated protein kinase in metabolic reprogramming of metastatic cancer cells: targeting cellular bioenergetics and biosynthetic pathways for anti-tumor treatment.J Physiol Pharmacol. 2018 Jun;69(3). doi: 10.26402/jpp.2018.3.07. Epub 2018 Sep 28. J Physiol Pharmacol. 2018. PMID: 30279304 Review.
-
Reactive oxygen species (ROS) are a key determinant of cancer's metabolic phenotype.Int J Cancer. 2018 Feb 1;142(3):440-448. doi: 10.1002/ijc.31069. Epub 2017 Oct 9. Int J Cancer. 2018. PMID: 28940517 Review.
Cited by
-
Trends in metabolic signaling pathways of tumor drug resistance: A scientometric analysis.Front Oncol. 2022 Oct 25;12:981406. doi: 10.3389/fonc.2022.981406. eCollection 2022. Front Oncol. 2022. PMID: 36387132 Free PMC article.
-
Heterozygous SOD2 deletion selectively impairs SERCA function in the soleus of female mice.Physiol Rep. 2022 May;10(10):e15285. doi: 10.14814/phy2.15285. Physiol Rep. 2022. PMID: 35581738 Free PMC article.
-
Targeting caseinolytic mitochondrial matrix peptidase, a novel contributor to the pathobiology of high-risk multiple myeloma.Blood. 2025 May 29;145(22):2614-2629. doi: 10.1182/blood.2024024781. Blood. 2025. PMID: 39912779
-
Adaptive changes in tumor cells in response to reductive stress.Biochem Pharmacol. 2024 Jan;219:115929. doi: 10.1016/j.bcp.2023.115929. Epub 2023 Nov 22. Biochem Pharmacol. 2024. PMID: 38000559 Free PMC article.
-
Oxidative Pentose Phosphate Pathway Enzyme 6-Phosphogluconate Dehydrogenase Plays a Key Role in Breast Cancer Metabolism.Biology (Basel). 2021 Jan 23;10(2):85. doi: 10.3390/biology10020085. Biology (Basel). 2021. PMID: 33498665 Free PMC article.
References
-
- Nakashima R.A., Paggi M.G., Pedersen P.L. Contributions of glycolysis and oxidative phosphorylation to adenosine 5′-triphosphate production in AS-30D hepatoma cells. Cancer Res. 1984;44:5702–5706. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

