Advances and Challenges in Developing Efficient Graphene Oxide-Based ZnO Photocatalysts for Dye Photo-Oxidation
- PMID: 32408530
- PMCID: PMC7279554
- DOI: 10.3390/nano10050932
Advances and Challenges in Developing Efficient Graphene Oxide-Based ZnO Photocatalysts for Dye Photo-Oxidation
Abstract
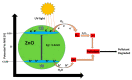
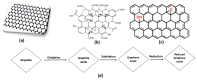
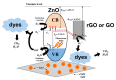
The efficient remediation of organic dyes from wastewater is increasingly valuable in water treatment technology, largely owing to the tons of hazardous chemicals currently and constantly released into rivers and seas from various industries, including the paper, pharmaceutical, textile, and dye production industries. Using solar energy as an inexhaustible source, photocatalysis ranks among the most promising wastewater treatment techniques for eliminating persistent organic pollutants and new emerging contaminants. In that context, developing efficient photocatalysts using sunlight irradiation and effectively integrating them into reactors, however, pose major challenges in the technologically relevant application of photocatalysts. As a potential solution, graphene oxide (GO)-based zinc oxide (ZnO) nanocomposites may be used together with different components (i.e., ZnO and GO-based materials) to overcome the drawbacks of ZnO photocatalysts. Indeed, mounting evidence suggests that using GO-based ZnO nanocomposites can promote light absorption, charge separation, charge transportation, and photo-oxidation of dyes. Despite such advances, viable, low-cost GO-based ZnO nanocomposite photocatalysts with sufficient efficiency, stability, and photostability remain to be developed, especially ones that can be integrated into photocatalytic reactors. This article offers a concise overview of state-of-the-art GO-based ZnO nanocomposites and the principal challenges in developing them.
Keywords: dye photodegradation; graphene oxide; photocatalysis; wastewater treatment; zinc oxide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Chan S.H.S., Wu T.Y., Juan J.C., Teh C.Y. Recent developments of metal oxide semiconductors as photocatalysts in advanced oxidation processes (AOPs) for treatment of dye waste-water. J. Chem. Technol. Biotechnol. 2011;86:1130–1158. doi: 10.1002/jctb.2636. - DOI
-
- Raizada P., Singh P., Kumar A., Pare B., Jonnalagadda S.B. Zero valent iron-brick grain nanocomposite for enhanced solar-Fenton removal of malachite green. Sep. Purif. Technol. 2014;133:429–437. doi: 10.1016/j.seppur.2014.07.012. - DOI
-
- Micheal K., Ayeshamariam A., Boddula R., Arunachalam P., AlSalhi M.S., Theerthagiri J., Prasad S., Madhavan J., Al-Mayouf A.M. Assembled composite of hematite iron oxide on sponge-like BiOCl with enhanced photocatalytic activity.Mater. Sci. Energy Technol. 2019;2:104–111.
-
- Raizada P., Sudhaik A., Singh P., Shandilya P., Thakur P., Jung H. Visible light assisted photodegradation of 2,4-dinitrophenol using Ag2CO3 loaded phosphorus and sulphur co-doped graphitic carbon nitride nanosheets in simulated wastewater. Arab. J. Chem. 2020;13:3196–3209. doi: 10.1016/j.arabjc.2018.10.004. - DOI
-
- Raizada P., Sudhaik A., Singh P., Shandilya P., Saini A.K., Gupta V.K., Lim J.H., Jung H., Hosseini-Bandegharaei A. Fabrication of Ag3VO4 decorated phosphorus and sulphur co-doped graphitic carbon nitride as a high-dispersed photocatalyst for phenol mineralization and E. coli disinfection. Sep. Purif. Technol. 2019;212:887–900. doi: 10.1016/j.seppur.2018.12.007. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

