Effect of Poly(vinyl alcohol) on Nanoencapsulation of Budesonide in Chitosan Nanoparticles via Ionic Gelation and Its Improved Bioavailability
- PMID: 32408557
- PMCID: PMC7285374
- DOI: 10.3390/polym12051101
Effect of Poly(vinyl alcohol) on Nanoencapsulation of Budesonide in Chitosan Nanoparticles via Ionic Gelation and Its Improved Bioavailability
Abstract
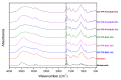
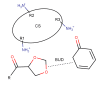
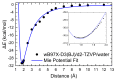
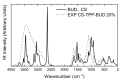
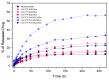
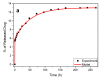
Chitosan (CS) is a polymer extensively used in drug delivery formulations mainly due to its biocompatibility and low toxicity. In the present study, chitosan was used for nanoencapsulation of a budesonide (BUD) drug via the well-established ionic gelation technique and a slight modification of it, using also poly(vinyl alcohol) (PVA) as a surfactant. Scanning electron microscopy (SEM) micrographs revealed that spherical nanoparticles were successfully prepared with average sizes range between 363 and 543 nm, as were measured by dynamic light scattering (DLS), while zeta potential verified their positive charged surface. X-ray diffraction (XRD) patterns revealed that BUD was encapsulated in crystalline state in nanoparticles but with a lower degree of crystallinity than the neat drug, which was also proven by differential scanning calorimetry (DSC) and melting peak measurements. This could be attributed to interactions that take place between BUD and CS, which were revealed by FTIR and by an extended computational study. An in vitro release study of budesonide showed a slight enhancement in the BUD dissolution profile, compared to the neat drug. However, drug release was substantially increased by introducing PVA during the nanoencapsulation procedure, which is attributed to the higher amorphization of BUD on these nanoparticles. The release curves were analyzed using a diffusion model that allows estimation of BUD diffusivity in the nanoparticles.
Keywords: COPD treatment; budesonide; chitosan nanoparticles; drug dissolution enhancement; drug release; sustain release.
Conflict of interest statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Figures
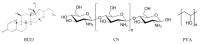






Similar articles
-
Microencapsulation of Fluticasone Propionate and Salmeterol Xinafoate in Modified Chitosan Microparticles for Release Optimization.Molecules. 2020 Aug 26;25(17):3888. doi: 10.3390/molecules25173888. Molecules. 2020. PMID: 32859128 Free PMC article.
-
Chitosan and chitosan/PEG nanoparticles loaded with indole-3-carbinol: Characterization, computational study and potential effect on human bladder cancer cells.Mater Sci Eng C Mater Biol Appl. 2021 May;124:112089. doi: 10.1016/j.msec.2021.112089. Epub 2021 Mar 31. Mater Sci Eng C Mater Biol Appl. 2021. PMID: 33947529
-
Alginate coated chitosan core shell nanoparticles for oral delivery of enoxaparin: in vitro and in vivo assessment.Int J Pharm. 2013 Nov 1;456(1):31-40. doi: 10.1016/j.ijpharm.2013.08.037. Epub 2013 Aug 29. Int J Pharm. 2013. PMID: 23994363
-
Budesonide nanocrystal-loaded hyaluronic acid microparticles for inhalation: In vitro and in vivo evaluation.Carbohydr Polym. 2018 Feb 1;181:1143-1152. doi: 10.1016/j.carbpol.2017.11.018. Epub 2017 Nov 4. Carbohydr Polym. 2018. PMID: 29253943
-
Chitosan Derivatives with Mucoadhesive and Antimicrobial Properties for Simultaneous Nanoencapsulation and Extended Ocular Release Formulations of Dexamethasone and Chloramphenicol Drugs.Pharmaceutics. 2020 Jun 26;12(6):594. doi: 10.3390/pharmaceutics12060594. Pharmaceutics. 2020. PMID: 32604758 Free PMC article.
Cited by
-
A Water-Soluble Chitosan Derivative for the Release of Bioactive Deferoxamine.Int J Mol Sci. 2024 Jan 11;25(2):913. doi: 10.3390/ijms25020913. Int J Mol Sci. 2024. PMID: 38255991 Free PMC article.
-
Microencapsulation of Fluticasone Propionate and Salmeterol Xinafoate in Modified Chitosan Microparticles for Release Optimization.Molecules. 2020 Aug 26;25(17):3888. doi: 10.3390/molecules25173888. Molecules. 2020. PMID: 32859128 Free PMC article.
-
Innovative Skin Product Emulsions with Enhanced Antioxidant, Antimicrobial and UV Protection Properties Containing Nanoparticles of Pure and Modified Chitosan with Encapsulated Fresh Pomegranate Juice.Polymers (Basel). 2020 Jul 12;12(7):1542. doi: 10.3390/polym12071542. Polymers (Basel). 2020. PMID: 32664701 Free PMC article.
-
Preparation and Characterization of a Novel Multiparticulate Dosage Form Carrying Budesonide-Loaded Chitosan Nanoparticles to Enhance the Efficiency of Pellets in the Colon.Pharmaceutics. 2022 Dec 26;15(1):69. doi: 10.3390/pharmaceutics15010069. Pharmaceutics. 2022. PMID: 36678698 Free PMC article.
-
Chitosan and its Derivatives for Ocular Delivery Formulations: Recent Advances and Developments.Polymers (Basel). 2020 Jul 8;12(7):1519. doi: 10.3390/polym12071519. Polymers (Basel). 2020. PMID: 32650536 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

