Experimental Comparative Study of Dynamic Behavior in Solution Phase of C-Tetra(phenyl)resorcin[4]arene and C-Tetra(phenyl)pyrogallol[4]arene
- PMID: 32408559
- PMCID: PMC7287697
- DOI: 10.3390/molecules25102275
Experimental Comparative Study of Dynamic Behavior in Solution Phase of C-Tetra(phenyl)resorcin[4]arene and C-Tetra(phenyl)pyrogallol[4]arene
Abstract
The synthesis of phenyl-resorcinarenes and pyrogallolarenes is known to produce a conformational mixture of cone and chair isomers. Depending on the synthesis conditions the composition of the conformational mixture is variable; however, the cone conformer is the greatest proportion of phenyl-resorcin[4]arenes and chair conformer of pyrogallol[4]arenes. The experimental evidence suggests that phenyl-substituted resorcinarene and pyrogallolarene exist as a dynamic boat in solution.
Keywords: conformational behavior; conformers; dynamic boat; dynamic studies; polyhydroxylated platform.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




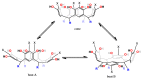
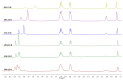
References
-
- Scott M., Sherburn M. Comprehensive Supramolecular Chemistry II. Elsevier; Amsterdam, The Netherlands: 2017. Resorcinarenes and Pyrogallolarenes; pp. 337–374. - DOI
-
- Han J., Song X., Liu L., Yan C. Synthesis, crystal structure and configuration of acetylated aryl Pyrogallol[4]arenes. J. Incl. Phenom. Macrocycl. Chem. 2007;59:257–263. doi: 10.1007/s10847-007-9323-2. - DOI
-
- Pfeiffer C., Feaster K.A., Dalgarno S.J., Atwood J. Syntheses and characterization of aryl-substituted pyrogallol[4]arenes and resorcin[4]arenes. CrystEngComm. 2016;18:222–229. doi: 10.1039/C5CE01792K. - DOI
-
- Jain V.K., Kanaiya P.H. Chemistry of calix[4]resorcinarenes. Russ. Chem. Rev. 2011;80:75–102. doi: 10.1070/RC2011v080n01ABEH004127. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

