Progerin Expression Induces Inflammation, Oxidative Stress and Senescence in Human Coronary Endothelial Cells
- PMID: 32408587
- PMCID: PMC7290406
- DOI: 10.3390/cells9051201
Progerin Expression Induces Inflammation, Oxidative Stress and Senescence in Human Coronary Endothelial Cells
Abstract
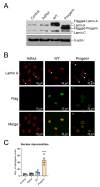
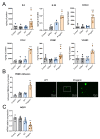
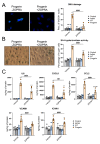
Hutchinson-Gilford progeria syndrome (HGPS) is a rare premature aging disorder notably characterized by precocious and deadly atherosclerosis. Almost 90% of HGPS patients carry a LMNA p.G608G splice variant that leads to the expression of a permanently farnesylated abnormal form of prelamin-A, referred to as progerin. Endothelial dysfunction is a key determinant of atherosclerosis, notably during aging. Previous studies have shown that progerin accumulates in HGPS patients' endothelial cells but also during vascular physiological aging. However, whether progerin expression in human endothelial cells can recapitulate features of endothelial dysfunction is currently unknown. Herein, we evaluated the direct impact of exogenously expressed progerin and wild-type lamin-A on human endothelial cell function and senescence. Our data demonstrate that progerin, but not wild-type lamin-A, overexpression induces endothelial cell dysfunction, characterized by increased inflammation and oxidative stress together with persistent DNA damage, increased cell cycle arrest protein expression and cellular senescence. Inhibition of progerin prenylation using a pravastatin-zoledronate combination partly prevents these defects. Our data suggest a direct proatherogenic role of progerin in human endothelial cells, which could contribute to HGPS-associated early atherosclerosis and also potentially be involved in physiological endothelial aging participating to age-related cardiometabolic diseases.
Keywords: Hutchinson–Gilford progeria syndrome; LMNA; aging; atherosclerosis; endothelial dysfunction; inflammation; lamin A; prenylation; progerin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

