Treatment Motivations and Expectations in Patients with Actinic Keratosis: A German-Wide Multicenter, Cross-Sectional Trial
- PMID: 32408601
- PMCID: PMC7290787
- DOI: 10.3390/jcm9051438
Treatment Motivations and Expectations in Patients with Actinic Keratosis: A German-Wide Multicenter, Cross-Sectional Trial
Abstract
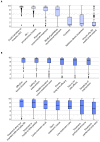
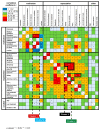
Patient-centered motives and expectations of the treatment of actinic keratoses (AK) have received little attention until now. Hence, we aimed to profile and cluster treatment motivations and expectations among patients with AK in a nationwide multicenter, cross-sectional study including patients from 14 German skin cancer centers. Patients were asked to complete a self-administered questionnaire. Treatment motives and expectations towards AK management were measured on a visual analogue scale from 1-10. Specific patient profiles were investigated with subgroup and correlation analysis. Overall, 403 patients were included. The highest motivation values were obtained for the items "avoid transition to invasive squamous cell carcinoma" (mean ± standard deviation; 8.98 ± 1.46), "AK are considered precancerous lesions" (8.72 ± 1.34) and "treating physician recommends treatment" (8.10 ± 2.37; p < 0.0001). The highest expectation values were observed for the items "effective lesion clearance" (8.36 ± 1.99), "safety" (8.20 ± 2.03) and "treatment-related costs are covered by health insurance" (8.00 ± 2.41; p < 0.0001). Patients aged ≥77 years and those with ≥7 lesions were identified at high risk of not undergoing any treatment due to intrinsic and extrinsic motivation deficits. Heat mapping of correlation analysis revealed four clusters with distinct motivation and expectation profiles. This study provides a patient-based heuristic tool for a personalized treatment decision in patients with AK.
Keywords: actinic keratosis; cross-sectional study; patient education; patient-centered care; patient-reported outcomes; personalized medicine; skin cancer.
Conflict of interest statement
C.B. has been a member of advisory boards for Almirall Hermal, Biofrontera, Galderma, ISDIN, and Leo Pharma, has received speaker’s honoraria by Almirall Hermal, Galderma, and Leo Pharma, and has received funding for clinical research by Leo Pharma. M.V.H. has been a member of advisory boards for Almirall Hermal and received speaker’s honoraria by Galderma and Biofrontera. K.C.K. has been a member of advisory boards for Almirall Hermal and has received speaker’s honoraria by Almirall Hermal. U.L. has been a member of advisory boards for Sanofi, Roche and Novartis and has received speaker’s honoraria by MSD, Novartis, Roche, Sanofi. L.S. has been a member of advisory boards for Almirall Hermal and received speaker’s honoraria by Almirall Hermal, Leo Pharma, Mylan, Galderma and Biofrontera. J.U. is on the advisory board or has received honoraria and travel support from Amgen, Bristol Myers Squibb, GSK, LeoPharma, Merck Sharp and Dohme, Novartis, Pierre Fabre, Roche, Sanofi outside the submitted work. S.K. has participated in previous clinical trials for Galderma and Leo Pharma. A.Z. has been a member of advisory boards for Beiersdorf Dermo Medical, Galderma, and has received speaker’s honoraria by Almirall Hermal, Beiersdorf Dermo Medical, Galderma and has received funding for clinical research by Beiersdorf Dermo Medical, Galderma. The remaining authors declare no conflicts of interests.
Figures

References
-
- Criscione V.D., Weinstock M.A., Naylor M.F., Luque C., Eide M.J., Bingham S.F., for the Department of Veteran Affairs Topical Tretinoin Chemoprevention Trial Group Actinic keratoses: Natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523–2530. doi: 10.1002/cncr.24284. - DOI - PubMed
LinkOut - more resources
Full Text Sources

