TRPV1: Structure, Endogenous Agonists, and Mechanisms
- PMID: 32408609
- PMCID: PMC7279265
- DOI: 10.3390/ijms21103421
TRPV1: Structure, Endogenous Agonists, and Mechanisms
Abstract
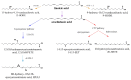
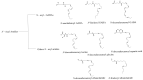
The Transient Receptor Potential Vanilloid 1 (TRPV1) channel is a polymodal protein with functions widely linked to the generation of pain. Several agonists of exogenous and endogenous nature have been described for this ion channel. Nonetheless, detailed mechanisms and description of binding sites have been resolved only for a few endogenous agonists. This review focuses on summarizing discoveries made in this particular field of study and highlighting the fact that studying the molecular details of activation of the channel by different agonists can shed light on biophysical traits that had not been previously demonstrated.
Keywords: TRP channels; TRPV1; agonist; pain; structure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

