Diffusion Tensor Cardiovascular Magnetic Resonance in Cardiac Amyloidosis
- PMID: 32408830
- PMCID: PMC7255887
- DOI: 10.1161/CIRCIMAGING.119.009901
Diffusion Tensor Cardiovascular Magnetic Resonance in Cardiac Amyloidosis
Abstract
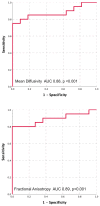
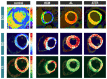
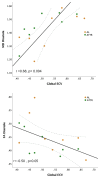
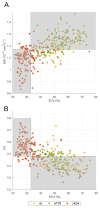
Background Cardiac amyloidosis (CA) is a disease of interstitial myocardial infiltration, usually by light chains or transthyretin. We used diffusion tensor cardiovascular magnetic resonance (DT-CMR) to noninvasively assess the effects of amyloid infiltration on the cardiac microstructure. Methods DT-CMR was performed at diastole and systole in 20 CA, 11 hypertrophic cardiomyopathy, and 10 control subjects with calculation of mean diffusivity, fractional anisotropy, and sheetlet orientation (secondary eigenvector angle). Results Mean diffusivity was elevated and fractional anisotropy reduced in CA compared with both controls and hypertrophic cardiomyopathy (P<0.001). In CA, mean diffusivity was correlated with extracellular volume (r=0.68, P=0.004), and fractional anisotropy was inversely correlated with circumferential strain (r=-0.65, P=0.02). In CA, diastolic secondary eigenvector angle was elevated, and secondary eigenvector angle mobility was reduced compared with controls (both P<0.001). Diastolic secondary eigenvector angle was correlated with amyloid burden measured by extracellular volume in transthyretin, but not light chain amyloidosis. Conclusions DT-CMR can characterize the microstructural effects of amyloid infiltration and is a contrast-free method to identify the location and extent of the expanded disorganized myocardium. The diffusion biomarkers mean diffusivity and fractional anisotropy effectively discriminate CA from hypertrophic cardiomyopathy. DT-CMR demonstrated that failure of sheetlet relaxation in diastole correlated with extracellular volume in transthyretin, but not light chain amyloidosis. This indicates that different mechanisms may be responsible for impaired contractility in CA, with an amyloid burden effect in transthyretin, but an idiosyncratic effect in light chain amyloidosis. Consequently, DT-CMR offers a contrast-free tool to identify novel pathophysiology, improve diagnostics, and monitor disease through noninvasive microstructural assessment.
Keywords: amyloid; diffusion tensor imaging; extracellular space; magnetic resonance imaging; myocardium.
Conflict of interest statement
Professors Dudley Pennell and David Firmin receive research support from Siemens. Professor Pennell also has a research grant from La Jolla Pharma, and a research grant and consultancy with ApoPharma. All the other authors have no conflict of interest to declare.
Figures


Comment in
-
Phenotyping of Cardiac Amyloidosis: Advancing From Macro to Micro?Circ Cardiovasc Imaging. 2020 May;13(5):e010785. doi: 10.1161/CIRCIMAGING.120.010785. Epub 2020 May 15. Circ Cardiovasc Imaging. 2020. PMID: 32408832 Free PMC article. No abstract available.
Similar articles
-
Myocardial Amyloidosis: The Exemplar Interstitial Disease.JACC Cardiovasc Imaging. 2019 Nov;12(11 Pt 2):2345-2356. doi: 10.1016/j.jcmg.2019.06.023. Epub 2019 Aug 14. JACC Cardiovasc Imaging. 2019. PMID: 31422120 Review.
-
Cardiac Structural and Functional Consequences of Amyloid Deposition by Cardiac Magnetic Resonance and Echocardiography and Their Prognostic Roles.JACC Cardiovasc Imaging. 2019 May;12(5):823-833. doi: 10.1016/j.jcmg.2018.02.016. Epub 2018 Apr 18. JACC Cardiovasc Imaging. 2019. PMID: 29680336
-
Characterizing cardiac involvement in amyloidosis using cardiovascular magnetic resonance diffusion tensor imaging.J Cardiovasc Magn Reson. 2019 Sep 5;21(1):56. doi: 10.1186/s12968-019-0563-2. J Cardiovasc Magn Reson. 2019. PMID: 31484544 Free PMC article.
-
Noncontrast Magnetic Resonance for the Diagnosis of Cardiac Amyloidosis.JACC Cardiovasc Imaging. 2020 Jan;13(1 Pt 1):69-80. doi: 10.1016/j.jcmg.2019.03.026. Epub 2019 Jun 12. JACC Cardiovasc Imaging. 2020. PMID: 31202744
-
Native T1 Mapping, Extracellular Volume Mapping, and Late Gadolinium Enhancement in Cardiac Amyloidosis: A Meta-Analysis.JACC Cardiovasc Imaging. 2020 Jun;13(6):1299-1310. doi: 10.1016/j.jcmg.2020.03.010. JACC Cardiovasc Imaging. 2020. PMID: 32498919 Free PMC article.
Cited by
-
Novel Insights into Non-Invasive Diagnostic Techniques for Cardiac Amyloidosis: A Critical Review.Diagnostics (Basel). 2024 Oct 9;14(19):2249. doi: 10.3390/diagnostics14192249. Diagnostics (Basel). 2024. PMID: 39410653 Free PMC article. Review.
-
A Review of Current and Evolving Imaging Techniques in Cardiac Amyloidosis.Curr Treat Options Cardiovasc Med. 2023 Mar;25(3):43-63. doi: 10.1007/s11936-023-00976-7. Epub 2023 Mar 4. Curr Treat Options Cardiovasc Med. 2023. PMID: 38239280 Free PMC article.
-
Optimized New Shengmai Powder modulation of cAMP/Rap1A signaling pathway attenuates myocardial fibrosis in heart failure.Chin Med. 2024 Feb 24;19(1):30. doi: 10.1186/s13020-024-00902-4. Chin Med. 2024. PMID: 38402401 Free PMC article.
-
Diffusion-weighted imaging and diffusion tensor imaging of the heart in vivo: major developments.Postepy Kardiol Interwencyjnej. 2022 Dec;18(4):350-359. doi: 10.5114/aic.2022.121345. Epub 2022 Nov 19. Postepy Kardiol Interwencyjnej. 2022. PMID: 36967858 Free PMC article. Review.
-
Ultra-high field cardiac MRI in large animals and humans for translational cardiovascular research.Front Cardiovasc Med. 2023 May 15;10:1068390. doi: 10.3389/fcvm.2023.1068390. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37255709 Free PMC article.
References
-
- González-López E, Gallego-Delgado M, Guzzo-Merello G, Moral de H, Javier F, Cobo-Marcos M, Robles C, Bornstein B, Salas C, Lara-Pezzi E, et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015;36:2585–94. doi: 10.1093/eurheartj/ehv338. - DOI - PubMed
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

