Anti-IL-20 antibody improved motor function and reduced glial scar formation after traumatic spinal cord injury in rats
- PMID: 32408881
- PMCID: PMC7227062
- DOI: 10.1186/s12974-020-01814-4
Anti-IL-20 antibody improved motor function and reduced glial scar formation after traumatic spinal cord injury in rats
Abstract
Background: Spinal cord injury (SCI) causes devastating neurological consequences, which can result in partial or total paralysis. Irreversible neurological deficits and glial scar formation are characteristic of SCI. Inflammatory responses are a major component of secondary injury and play a central role in regulating the pathogenesis of SCI. IL-20 is a proinflammatory cytokine involved in renal fibrosis and liver cirrhosis through its role in upregulating TGF-β1 production. However, the role of IL-20 in SCI remains unclear. We hypothesize that IL-20 is upregulated after SCI and is involved in regulating the neuroinflammatory response.
Methods: The expression of IL-20 and its receptors was examined in SCI rats. The regulatory roles of IL-20 in astrocytes and neuron cells were examined. The therapeutic effects of anti-IL-20 monoclonal antibody (mAb) 7E in SCI rats were evaluated.
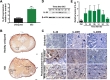
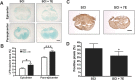
Results: Immunofluorescence staining showed that IL-20 and its receptors were expressed in astrocytes, oligodendrocytes, and microglia in the spinal cord after SCI in rats. In vitro, IL-20 enhanced astrocyte reactivation and cell migration in human astrocyte (HA) cells by upregulating glial fibrillary acidic protein (GFAP), TGF-β1, TNF-α, MCP-1, and IL-6 expression. IL-20 inhibited cell proliferation and nerve growth factor (NGF)-derived neurite outgrowth in PC-12 cells through Sema3A/NRP-1 upregulation. In vivo, treating SCI rats with anti-IL-20 mAb 7E remarkably inhibited the inflammatory responses. 7E treatment not only improved motor and sensory functions but also improved spinal cord tissue preservation and reduced glial scar formation in SCI rats.
Conclusions: IL-20 might regulate astrocyte reactivation and axonal regeneration and result in the secondary injury in SCI. These findings demonstrated that IL-20 may be a promising target for SCI treatment.
Keywords: IL-20; Neuroinflammation; SCI.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Tator CH, Duncan EG, Edmonds VE, Lapczak LI, Andrews DF. Neurological recovery, mortality and length of stay after acute spinal cord injury associated with changes in management. Paraplegia. 1995;33:254–262. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

