Contemporary resuscitation of hemorrhagic shock: What will the future hold?
- PMID: 32409009
- PMCID: PMC7211588
- DOI: 10.1016/j.amjsurg.2020.05.008
Contemporary resuscitation of hemorrhagic shock: What will the future hold?
Abstract
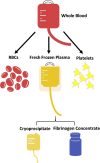
Resuscitation of the critically ill patient with fluid and blood products is one of the most widespread interventions in medicine. This is especially relevant for trauma patients, as hemorrhagic shock remains the most common cause of preventable death after injury. Consequently, the study of the ideal resuscitative product for patients in shock has become an area of great scientific interest and investigation. Recently, the pendulum has swung towards increased utilization of blood products for resuscitation. However, pathogens, immune reactions and the limited availability of this resource remain a challenge for clinicians. Technologic advances in pathogen reduction and innovations in blood product processing will allow us to increase the safety profile and efficacy of blood products, ultimately to the benefit of patients. The purpose of this article is to review the current state of blood product based resuscitative strategies as well as technologic advancements that may lead to safer resuscitation.
Keywords: Cryoprecipitate; Crystalloid; Fibrinogen; Fresh frozen plasma; Hemorrhagic shock; Intercept; Mirasol; Octaplas; Platelets; Resuscitation; Whole blood.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures
References
-
- Deaths C.D.C. US Department of; CDC; National Center for Health Statistics.; 2010. Final Data for 2009.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

