Arginine Depletion Therapy with ADI-PEG20 Limits Tumor Growth in Argininosuccinate Synthase-Deficient Ovarian Cancer, Including Small-Cell Carcinoma of the Ovary, Hypercalcemic Type
- PMID: 32409304
- PMCID: PMC7442649
- DOI: 10.1158/1078-0432.CCR-19-1905
Arginine Depletion Therapy with ADI-PEG20 Limits Tumor Growth in Argininosuccinate Synthase-Deficient Ovarian Cancer, Including Small-Cell Carcinoma of the Ovary, Hypercalcemic Type
Abstract
Purpose: Many rare ovarian cancer subtypes, such as small-cell carcinoma of the ovary, hypercalcemic type (SCCOHT), have poor prognosis due to their aggressive nature and resistance to standard platinum- and taxane-based chemotherapy. The development of effective therapeutics has been hindered by the rarity of such tumors. We sought to identify targetable vulnerabilities in rare ovarian cancer subtypes.
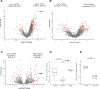
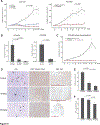
Experimental design: We compared the global proteomic landscape of six cases each of endometrioid ovarian cancer (ENOC), clear cell ovarian cancer (CCOC), and SCCOHT to the most common subtype, high-grade serous ovarian cancer (HGSC), to identify potential therapeutic targets. IHC of tissue microarrays was used as validation of arginosuccinate synthase (ASS1) deficiency. The efficacy of arginine-depriving therapeutic ADI-PEG20 was assessed in vitro using cell lines and patient-derived xenograft mouse models representing SCCOHT.
Results: Global proteomic analysis identified low ASS1 expression in ENOC, CCOC, and SCCOHT compared with HGSC. Low ASS1 levels were validated through IHC in large patient cohorts. The lowest levels of ASS1 were observed in SCCOHT, where ASS1 was absent in 12 of 31 cases, and expressed in less than 5% of the tumor cells in 9 of 31 cases. ASS1-deficient ovarian cancer cells were sensitive to ADI-PEG20 treatment regardless of subtype in vitro. Furthermore, in two cell line mouse xenograft models and one patient-derived mouse xenograft model of SCCOHT, once-a-week treatment with ADI-PEG20 (30 mg/kg and 15 mg/kg) inhibited tumor growth in vivo.
Conclusions: Preclinical in vitro and in vivo studies identified ADI-PEG20 as a potential therapy for patients with rare ovarian cancers, including SCCOHT.
©2020 American Association for Cancer Research.
Conflict of interest statement
A conflict of interest disclosure statement
The authors do not have any conflict of interest.
Figures



Similar articles
-
Bench-to-Bedside Studies of Arginine Deprivation in Cancer.Molecules. 2023 Feb 24;28(5):2150. doi: 10.3390/molecules28052150. Molecules. 2023. PMID: 36903394 Free PMC article. Review.
-
Efficacy of arginine depletion by ADI-PEG20 in an intracranial model of GBM.Cell Death Dis. 2018 Dec 13;9(12):1192. doi: 10.1038/s41419-018-1195-4. Cell Death Dis. 2018. PMID: 30546006 Free PMC article.
-
ASS1 as a novel tumor suppressor gene in myxofibrosarcomas: aberrant loss via epigenetic DNA methylation confers aggressive phenotypes, negative prognostic impact, and therapeutic relevance.Clin Cancer Res. 2013 Jun 1;19(11):2861-72. doi: 10.1158/1078-0432.CCR-12-2641. Epub 2013 Apr 2. Clin Cancer Res. 2013. PMID: 23549872
-
A role for macrophages under cytokine control in mediating resistance to ADI-PEG20 (pegargiminase) in ASS1-deficient mesothelioma.Pharmacol Rep. 2023 Jun;75(3):570-584. doi: 10.1007/s43440-023-00480-6. Epub 2023 Apr 3. Pharmacol Rep. 2023. PMID: 37010783 Free PMC article.
-
Targeted cellular metabolism for cancer chemotherapy with recombinant arginine-degrading enzymes.Oncotarget. 2010 Aug;1(4):246-51. doi: 10.18632/oncotarget.135. Oncotarget. 2010. PMID: 21152246 Free PMC article. Review.
Cited by
-
Amino acid metabolism in tumor biology and therapy.Cell Death Dis. 2024 Jan 13;15(1):42. doi: 10.1038/s41419-024-06435-w. Cell Death Dis. 2024. PMID: 38218942 Free PMC article. Review.
-
Bench-to-Bedside Studies of Arginine Deprivation in Cancer.Molecules. 2023 Feb 24;28(5):2150. doi: 10.3390/molecules28052150. Molecules. 2023. PMID: 36903394 Free PMC article. Review.
-
Long noncoding RNA LINC01234 promotes hepatocellular carcinoma progression through orchestrating aspartate metabolic reprogramming.Mol Ther. 2022 Jun 1;30(6):2354-2369. doi: 10.1016/j.ymthe.2022.02.020. Epub 2022 Feb 19. Mol Ther. 2022. PMID: 35192933 Free PMC article.
-
Research progress on the role of cationic amino acid transporter (CAT) family members in malignant tumors and immune microenvironment.Amino Acids. 2023 Oct;55(10):1213-1222. doi: 10.1007/s00726-023-03313-1. Epub 2023 Aug 12. Amino Acids. 2023. PMID: 37572157 Review.
-
Barriers to Immunotherapy in Ovarian Cancer: Metabolic, Genomic, and Immune Perturbations in the Tumour Microenvironment.Cancers (Basel). 2021 Dec 11;13(24):6231. doi: 10.3390/cancers13246231. Cancers (Basel). 2021. PMID: 34944851 Free PMC article. Review.
References
-
- Karnezis AN, Cho KR, Gilks CB, Pearce CL, Huntsman DG. The disparate origins of ovarian cancers: pathogenesis and prevention strategies. Nat Rev Cancer. 2017;17(1):65–74. - PubMed
-
- Pujade-Lauraine E, Ledermann JA, Selle F, Gebski V, Penson RT, Oza AM, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA½ mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(9):1274–84. - PubMed
-
- Sato N, Tsunoda H, Nishida M, Morishita Y, Takimoto Y, Kubo T, et al. Loss of heterozygosity on 10q23.3 and mutation of the tumor suppressor gene PTEN in benign endometrial cyst of the ovary: possible sequence progression from benign endometrial cyst to endometrioid carcinoma and clear cell carcinoma of the ovary. Cancer Res. 2000;60(24):7052–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

