Diverse functionalization of strong alkyl C-H bonds by undirected borylation
- PMID: 32409470
- PMCID: PMC7710342
- DOI: 10.1126/science.aba6146
Diverse functionalization of strong alkyl C-H bonds by undirected borylation
Abstract
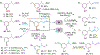
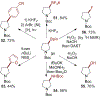
The selective functionalization of strong, typically inert carbon-hydrogen (C-H) bonds in organic molecules is changing synthetic chemistry. However, the undirected functionalization of primary C-H bonds without competing functionalization of secondary C-H bonds is rare. The borylation of alkyl C-H bonds has occurred previously with this selectivity, but slow rates required the substrate to be the solvent or in large excess. We report an iridium catalyst ligated by 2-methylphenanthroline with activity that enables, with the substrate as limiting reagent, undirected borylation of primary C-H bonds and, when primary C-H bonds are absent or blocked, borylation of strong secondary C-H bonds. Reactions at the resulting carbon-boron bond show how these borylations can lead to the installation of a wide range of carbon-carbon and carbon-heteroatom bonds at previously inaccessible positions of organic molecules.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures


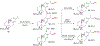
References
-
- Arndtsen BA, Bergman RG, Mobley TA, Peterson TH, Acc. Chem. Res. 28, 154–162 (1995).

