Randomized phase 2 study of adjunctive cenobamate in patients with uncontrolled focal seizures
- PMID: 32409485
- PMCID: PMC7357293
- DOI: 10.1212/WNL.0000000000009530
Randomized phase 2 study of adjunctive cenobamate in patients with uncontrolled focal seizures
Abstract
Objective: To evaluate the efficacy and safety of adjunctive cenobamate 200 mg/d in patients with uncontrolled focal (partial-onset) seizures despite treatment with 1 to 3 antiepileptic drugs.
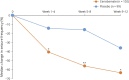
Methods: In this multicenter, double-blind, placebo-controlled study, adults 18 to 65 years of age with focal seizures were randomized 1:1 (cenobamate:placebo) after an 8-week baseline period. The 12-week double-blind treatment period consisted of a 6-week titration phase and a 6-week maintenance phase. The primary outcome was percent change in seizure frequency (from baseline) per 28 days during double-blind treatment.
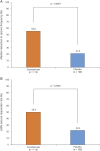
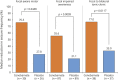
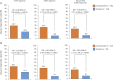
Results: Two hundred twenty-two patients were randomized; 113 received cenobamate and 109 received placebo; and 90.3% and 90.8% of patients, respectively, completed double-blind treatment. Median baseline seizure frequency was 6.5 in 28 days (range 0-237). Compared to placebo, cenobamate conferred a greater median percent seizure reduction (55.6% vs 21.5%; p < 0.0001) The responder rate (≥50% reduction in seizure frequency) was 50.4% for cenobamate and 22.2% for placebo (p < 0.0001). Focal seizures with motor component, impaired awareness, and focal to bilateral tonic-clonic seizures were significantly reduced with cenobamate vs placebo. During maintenance, 28.3% of cenobamate-treated and 8.8% of placebo-treated patients were seizure-free. Treatment-emergent adverse events reported in >10% in either group (cenobamate vs placebo) were somnolence (22.1% vs 11.9%), dizziness (22.1% vs 16.5%), headache (12.4% vs 12.8%), nausea (11.5% vs 4.6%), and fatigue (10.6% vs 6.4%).
Conclusion: Adjunctive treatment with cenobamate 200 mg/d significantly improved seizure control in adults with uncontrolled focal seizures and was well tolerated.
Clinicaltrialsgov identifier: NCT01397968.
Classification of evidence: This study provides Class I evidence that, for patients with uncontrolled focal seizures, adjunctive cenobamate reduces seizures.
Copyright © 2020 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures

References
-
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med 2000;342:314–319. - PubMed
-
- Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS. Progress report on new antiepileptic drugs: a summary of the Eleventh Eilat Conference (EILAT XI). Epilepsy Res 2013;103:2–30. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
