Efficacy and safety of corticosteroids in COVID-19 based on evidence for COVID-19, other coronavirus infections, influenza, community-acquired pneumonia and acute respiratory distress syndrome: a systematic review and meta-analysis
- PMID: 32409522
- PMCID: PMC7828900
- DOI: 10.1503/cmaj.200645
Efficacy and safety of corticosteroids in COVID-19 based on evidence for COVID-19, other coronavirus infections, influenza, community-acquired pneumonia and acute respiratory distress syndrome: a systematic review and meta-analysis
Abstract
Background: Very little direct evidence exists on use of corticosteroids in patients with coronavirus disease 2019 (COVID-19). Indirect evidence from related conditions must therefore inform inferences regarding benefits and harms. To support a guideline for managing COVID-19, we conducted systematic reviews examining the impact of corticosteroids in COVID-19 and related severe acute respiratory illnesses.
Methods: We searched standard international and Chinese biomedical literature databases and prepublication sources for randomized controlled trials (RCTs) and observational studies comparing corticosteroids versus no corticosteroids in patients with COVID-19, severe acute respiratory syndrome (SARS) or Middle East respiratory syndrome (MERS). For acute respiratory distress syndrome (ARDS), influenza and community-acquired pneumonia (CAP), we updated the most recent rigorous systematic review. We conducted random-effects meta-analyses to pool relative risks and then used baseline risk in patients with COVID-19 to generate absolute effects.
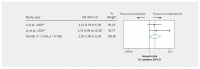
Results: In ARDS, according to 1 small cohort study in patients with COVID-19 and 7 RCTs in non-COVID-19 populations (risk ratio [RR] 0.72, 95% confidence interval [CI] 0.55 to 0.93, mean difference 17.3% fewer; low-quality evidence), corticosteroids may reduce mortality. In patients with severe COVID-19 but without ARDS, direct evidence from 2 observational studies provided very low-quality evidence of an increase in mortality with corticosteroids (hazard ratio [HR] 2.30, 95% CI 1.00 to 5.29, mean difference 11.9% more), as did observational data from influenza studies. Observational data from SARS and MERS studies provided very low-quality evidence of a small or no reduction in mortality. Randomized controlled trials in CAP suggest that corticosteroids may reduce mortality (RR 0.70, 95% CI 0.50 to 0.98, 3.1% lower; very low-quality evidence), and may increase hyperglycemia.
Interpretation: Corticosteroids may reduce mortality for patients with COVID-19 and ARDS. For patients with severe COVID-19 but without ARDS, evidence regarding benefit from different bodies of evidence is inconsistent and of very low quality.
© 2020 Joule Inc. or its licensors.
Conflict of interest statement
Competing interests: Bram Rochwerg is an investigator in a trial, supported by a Canadian Institute of Health Research grant, evaluating the effect of corticosteroids in COVID-19 patients. No other competing interests were declared.
Figures




Comment in
- CMAJ. 192:E536.
References
-
- WHO Director-General’s opening remarks at the media briefing on COVID-19 — 11 March 2020. Geneva: World Health Organization; 2020. Available: www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at... (accessed 2020 Apr. 23).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
