Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial
- PMID: 32409561
- PMCID: PMC7221473
- DOI: 10.1136/bmj.m1849
Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial
Abstract
Objective: To assess the efficacy and safety of hydroxychloroquine plus standard of care compared with standard of care alone in adults with coronavirus disease 2019 (covid-19).
Design: Multicentre, open label, randomised controlled trial.
Setting: 16 government designated covid-19 treatment centres in China, 11 to 29 February 2020.
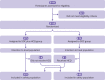
Participants: 150 patients admitted to hospital with laboratory confirmed covid-19 were included in the intention to treat analysis (75 patients assigned to hydroxychloroquine plus standard of care, 75 to standard of care alone).
Interventions: Hydroxychloroquine administrated at a loading dose of 1200 mg daily for three days followed by a maintenance dose of 800 mg daily (total treatment duration: two or three weeks for patients with mild to moderate or severe disease, respectively).
Main outcome measure: Negative conversion of severe acute respiratory syndrome coronavirus 2 by 28 days, analysed according to the intention to treat principle. Adverse events were analysed in the safety population in which hydroxychloroquine recipients were participants who received at least one dose of hydroxychloroquine and hydroxychloroquine non-recipients were those managed with standard of care alone.
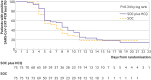
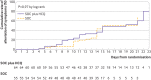
Results: Of 150 patients, 148 had mild to moderate disease and two had severe disease. The mean duration from symptom onset to randomisation was 16.6 (SD 10.5; range 3-41) days. A total of 109 (73%) patients (56 standard of care; 53 standard of care plus hydroxychloroquine) had negative conversion well before 28 days, and the remaining 41 (27%) patients (19 standard of care; 22 standard of care plus hydroxychloroquine) were censored as they did not reach negative conversion of virus. The probability of negative conversion by 28 days in the standard of care plus hydroxychloroquine group was 85.4% (95% confidence interval 73.8% to 93.8%), similar to that in the standard of care group (81.3%, 71.2% to 89.6%). The difference between groups was 4.1% (95% confidence interval -10.3% to 18.5%). In the safety population, adverse events were recorded in 7/80 (9%) hydroxychloroquine non-recipients and in 21/70 (30%) hydroxychloroquine recipients. The most common adverse event in the hydroxychloroquine recipients was diarrhoea, reported in 7/70 (10%) patients. Two hydroxychloroquine recipients reported serious adverse events.
Conclusions: Administration of hydroxychloroquine did not result in a significantly higher probability of negative conversion than standard of care alone in patients admitted to hospital with mainly persistent mild to moderate covid-19. Adverse events were higher in hydroxychloroquine recipients than in non-recipients.
Trial registration: ChiCTR2000029868.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work other than those listed above; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures
Comment in
-
Lack of efficacy of hydroxychloroquine in covid-19.BMJ. 2020 May 19;369:m2018. doi: 10.1136/bmj.m2018. BMJ. 2020. PMID: 32430461 No abstract available.
References
-
- Coronavirus covid-19 global cases by Johns Hopkins CSSE. https://www.arcgis.com/apps/opsdashboard/index.html#/85320e2ea5424dfaaa7....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
