Development of Small Molecule MEIS Inhibitors that modulate HSC activity
- PMID: 32409701
- PMCID: PMC7224207
- DOI: 10.1038/s41598-020-64888-3
Development of Small Molecule MEIS Inhibitors that modulate HSC activity
Abstract
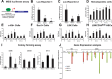
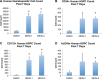
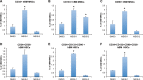
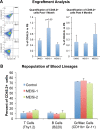
Meis1, which belongs to TALE-type class of homeobox gene family, appeared as one of the key regulators of hematopoietic stem cell (HSC) self-renewal and a potential therapeutical target. However, small molecule inhibitors of MEIS1 remained unknown. This led us to develop inhibitors of MEIS1 that could modulate HSC activity. To this end, we have established a library of relevant homeobox family inhibitors and developed a high-throughput in silico screening strategy against homeodomain of MEIS proteins using the AutoDock Vina and PaDEL-ADV platform. We have screened over a million druggable small molecules in silico and selected putative MEIS inhibitors (MEISi) with no predicted cytotoxicity or cardiotoxicity. This was followed by in vitro validation of putative MEIS inhibitors using MEIS dependent luciferase reporter assays and analysis in the ex vivo HSC assays. We have shown that small molecules named MEISi-1 and MEISi-2 significantly inhibit MEIS-luciferase reporters in vitro and induce murine (LSKCD34l°w cells) and human (CD34+, CD133+, and ALDHhi cells) HSC self-renewal ex vivo. In addition, inhibition of MEIS proteins results in downregulation of Meis1 and MEIS1 target gene expression including Hif-1α, Hif-2α and HSC quiescence modulators. MEIS inhibitors are effective in vivo as evident by induced HSC content in the murine bone marrow and downregulation of expression of MEIS target genes. These studies warrant identification of first-in-class MEIS inhibitors as potential pharmaceuticals to be utilized in modulation of HSC activity and bone marrow transplantation studies.
Conflict of interest statement
Dr. Fatih Kocabas is founder of Meinox Pharma Technologies. All other authors declare that they have no conflicts of interest concerning this work.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

