Early trends for SARS-CoV-2 infection in central and north Texas and impact on other circulating respiratory viruses
- PMID: 32410236
- PMCID: PMC7273053
- DOI: 10.1002/jmv.26010
Early trends for SARS-CoV-2 infection in central and north Texas and impact on other circulating respiratory viruses
Abstract
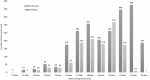
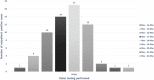
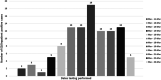
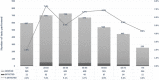
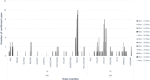
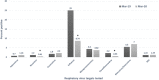
Rapid diagnosis and isolation are key to containing the quick spread of a pandemic agent like severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2), which has spread globally since its initial outbreak in Wuhan province in China. SARS-CoV-2 is novel and the effect on typically prevalent seasonal viruses is just becoming apparent. We present our initial data on the prevalence of respiratory viruses in the month of March 2020. This is a retrospective cohort study post launching of SARS-CoV-2 testing at Baylor Scott and White Hospital (BSWH), Temple, Texas. Testing for SARS-CoV-2 was performed by real-time reverse transcription polymerase chain reaction assay and results were shared with State public health officials for immediate interventions. More than 3500 tests were performed during the first 2 weeks of testing for SARS-CoV-2 and identified 168 (4.7%) positive patients. Sixty-two (3.2%) of the 1912 ambulatory patients and 106 (6.3%) of the 1659 emergency department/inpatients tested were positive. The highest rate of infection (6.9%) was seen in patients aged 25 to 34 years, while the lowest rate of infection was seen among patients aged <25 years old (2%). County-specific patient demographic information was shared with respective public health departments for epidemiological interventions. Incidentally, this study showed that there was a significant decrease in the occurrence of seasonal respiratory virus infections, perhaps due to increased epidemiological awareness about SARS-CoV-2 among the general public, as well as the social distancing measures implemented in response to SARS-CoV-2. Data extracted for BSWH from the Centers for Disease Control and Prevention's National Respiratory and Enteric Virus Surveillance System site revealed that Influenza incidence was 8.7% in March 2020, compared with 25% in March 2019. This study was intended to provide an initial experience of dealing with a pandemic and the role of laboratories in crisis management. This study provided SARS-CoV-2 testing data from ambulatory and inpatient population. Epidemiological interventions depend on timely availability of accurate diagnostic tests and throughput capacity of such systems during large outbreaks like SARS-CoV-2.
Keywords: COVID-19; SARS-COV-2; diagnosis; epidemiology; rRT-PCR.
© 2020 Wiley Periodicals LLC.
Conflict of interest statement
All authors declare that there are no conflict of interests.
Figures
References
-
- Coronavirus . https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019. Accessed 30 March 2020.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

