Surveillance of medically-attended influenza in elderly patients from Romania-data from three consecutive influenza seasons (2015/16, 2016/17, and 2017/18)
- PMID: 32410402
- PMCID: PMC7431641
- DOI: 10.1111/irv.12752
Surveillance of medically-attended influenza in elderly patients from Romania-data from three consecutive influenza seasons (2015/16, 2016/17, and 2017/18)
Abstract
Background: Influenza is an acute infection affecting all age groups; however, elderly patients are at an increased risk. We aim to describe the clinical characteristics and the circulation of influenza virus types in elderly patients admitted for severe acute respiratory infection (SARI) to a tertiary care hospital in Bucharest, Romania, part of the I-MOVE+ hospital network.
Methods: We conducted an active surveillance study at the National Institute for Infectious Diseases "Prof. Dr Matei Balș," Bucharest, Romania, during three consecutive influenza seasons: 2015/16, 2016/17, and 2017/18. All patients aged 65 and older admitted to our hospital for SARI were tested for influenza by PCR.
Results: A total of 349 eligible patients were tested during the study period, and 149 (42.7%) were confirmed with influenza. Most patients, 321 (92.5%) presented at least one underlying condition at the time of hospital admission, the most frequent being cardiovascular disease, 270 (78.3%). The main influenza viral subtype circulating in 2015/16 was A(H1N1)pdm09, followed by A(H3N2) in 2016/17 and B influenza in 2017/18. Case fatality was highest in the 2015/16 season (3.7%), 0% in 2016/17, and 1.0% in 2017/18. Vaccination coverage in elderly patients with SARI from our study population was 22 (6.3%) over the three seasons.
Conclusions: Our study has highlighted a high burden of comorbidities in elderly patients presenting with SARI during winter season in Romania. The influenza vaccine coverage rate needs to be substantially increased in the elderly population, through targeted interventions.
Keywords: case fatality; comorbidities; elderly; influenza; subtype.
© 2020 The Authors. Influenza and Other Respiratory Viruses Published by John Wiley & Sons Ltd.
Conflict of interest statement
DP is the technical project manager for the GIHSN project funded by Sanofi Pasteur and Foundation for Influenza Epidemiology, principal investigator of the I‐MOVE+ study funded through the European Union's HORIZON 2020 research and innovation program, and technical project manager for the DRIVE study, that has received support from the EU/EFPIA Innovative Medicines Initiative 2 Joint Undertaking (DRIVE, grant n° 777363). No conflict of interest.
AnSC is the principal investigator of the I‐MOVE+ study 2017/18 funded through the European Union's HORIZON 2020 research and innovation program, member of the research team of the DRIVE study, that has received support from the EU/EFPIA Innovative Medicines Initiative 2 Joint Undertaking (DRIVE, grant n° 777363), and subinvestigator in influenza clinical trials by Shionogi and Roche; no conflict of interest.
AEI is the coordinator of the molecular detection of influenza type and subtype by real‐time reverse transcription PCR through the IMOVE+ project. No conflict of interest.
ML is the coordinator of the genetic characterization of influenza strains. No conflict of interest.
CMC is the coordinator of the isolation of influenza viruses in cell culture and antigenic characterization in the IMOVE+ study. No conflict of interest.
MEM is the coordinator of the antiviral resistance in IMOVE+ study. No conflict of interest.
MN is the principal investigator of the I‐MOVE+ study 2016/18 funded through the European Union's HORIZON 2020 research and innovation program.
VA is the member of the research team of the GIHSN project funded by Sanofi Pasteur and Foundation for Influenza Epidemiology, member of the research team of the DRIVE study, that has received support from the EU/EFPIA Innovative Medicines Initiative 2 Joint Undertaking (DRIVE, grant n° 777363), and reports lectures and advisory boards for Sanofi and Astra‐Zeneca, outside of the submitted work. No conflict of interest.
MDC No conflict of interest.
ASC is the member of the research team of the GIHSN project funded by Sanofi Pasteur and Foundation for Influenza Epidemiology, member of the research team of the DRIVE study, that has received support from the EU/EFPIA Innovative Medicines Initiative 2 Joint Undertaking (DRIVE, grant n° 777363), principal investigator in influenza clinical trials by Shionogi and Roche, and reports lectures for Sanofi, outside of the submitted work. No conflict of interest.
OS is the member of the research team of the GIHSN project funded by Sanofi Pasteur and Foundation for Influenza Epidemiology, principal investigator for adults in the DRIVE study, that has received support from the EU/EFPIA Innovative Medicines Initiative 2 Joint Undertaking (DRIVE, grant n° 777363), subinvestigator in influenza clinical trials by Shionogi and Roche, and reports lectures for Sanofi, outside of the submitted work. No conflict of interest.
Figures

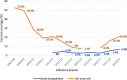
References
-
- Metodologia de supraveghere a gripei, infecţiilor respiratoriiacute (ARI) şi a infecţiilor respiratorii acute severe (SARI) pentru sezonul 2018‐2019. 2018. http://www.cnscbt.ro/index.php/metodologii/gripa‐infectii‐acute‐respirat.... Accessed August 9, 2019.
-
- Valenciano M, Ciancio B. I‐MOVE: a European network to measure the effectiveness of influenza vaccines. Euro Surveill. 2012;17(39). pii: 20281. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

