Empagliflozin in Heart Failure: Diuretic and Cardiorenal Effects
- PMID: 32410463
- PMCID: PMC7521417
- DOI: 10.1161/CIRCULATIONAHA.120.045691
Empagliflozin in Heart Failure: Diuretic and Cardiorenal Effects
Abstract
Background: Sodium-glucose cotransporter-2 inhibitors improve heart failure-related outcomes. The mechanisms underlying these benefits are not well understood, but diuretic properties may contribute. Traditional diuretics such as furosemide induce substantial neurohormonal activation, contributing to the limited improvement in intravascular volume often seen with these agents. However, the proximal tubular site of action of the sodium-glucose cotransporter-2 inhibitors may help circumvent these limitations.
Methods: Twenty patients with type 2 diabetes mellitus and chronic, stable heart failure completed a randomized, placebo-controlled crossover study of empagliflozin 10 mg daily versus placebo. Patients underwent an intensive 6-hour biospecimen collection and cardiorenal phenotyping at baseline and again after 14 days of study drug. After a 2-week washout, patients crossed over to the alternate therapy with the above protocol repeated.
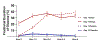
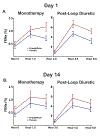
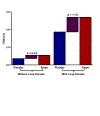
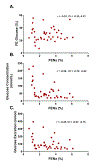
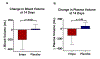
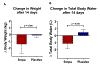
Results: Oral empagliflozin was rapidly absorbed as evidenced by a 27-fold increase in urinary glucose excretion by 3 hours (P<0.0001). Fractional excretion of sodium increased significantly with empagliflozin monotherapy versus placebo (fractional excretion of sodium, 1.2±0.7% versus 0.7±0.4%; P=0.001), and there was a synergistic effect in combination with bumetanide (fractional excretion of sodium, 5.8±2.5% versus 3.9±1.9%; P=0.001). At 14 days, the natriuretic effect of empagliflozin persisted, resulting in a reduction in blood volume (-208 mL [interquartile range, -536 to 153 mL] versus -14 mL [interquartile range, -282 to 335 mL]; P=0.035) and plasma volume (-138 mL, interquartile range, -379 to 154±453 mL; P=0.04). This natriuresis was not, however, associated with evidence of neurohormonal activation because the change in norepinephrine was superior (P=0.02) and all other neurohormones were similar (P<0.34) during the empagliflozin versus placebo period. Furthermore, there was no evidence of potassium wasting (P=0.20) or renal dysfunction (P>0.11 for all biomarkers), whereas both serum magnesium (P<0.001) and uric acid levels (P=0.008) improved.
Conclusions: Empagliflozin causes significant natriuresis, particularly when combined with loop diuretics, resulting in an improvement in blood volume. However, off-target electrolyte wasting, renal dysfunction, and neurohormonal activation were not observed. This favorable diuretic profile may offer significant advantage in the management of volume status in patients with heart failure and may represent a mechanism contributing to the superior long-term heart failure outcomes observed with these agents. Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT03027960.
Keywords: blood volume; natriuresis; sodium-glucose transporter 2 inhibitors.
Figures
Comment in
-
SGLT2 Inhibitor: Not a Traditional Diuretic for Heart Failure.Cell Metab. 2020 Jul 7;32(1):13-14. doi: 10.1016/j.cmet.2020.06.014. Cell Metab. 2020. PMID: 32640243
-
Canagliflozin independently reduced plasma volume from conventional diuretics in patients with type 2 diabetes and chronic heart failure: a subanalysis of the CANDLE trial.Hypertens Res. 2023 Feb;46(2):495-506. doi: 10.1038/s41440-022-01085-x. Epub 2022 Nov 15. Hypertens Res. 2023. PMID: 36380202
References
-
- Sarraf M, Masoumi A and Schrier RW. Cardiorenal syndrome in acute decompensated heart failure. Clinical journal of the American Society of Nephrology : CJASN. 2009;4:2013–2026. - PubMed
-
- Schrier RW. Use of diuretics in heart failure and cirrhosis. Semin Nephrol. 2011;31:503–12. - PubMed
-
- Schrier RW and Ecder T. Gibbs memorial lecture. Unifying hypothesis of body fluid volume regulation: implications for cardiac failure and cirrhosis. Mt Sinai J Med. 2001;68:350–61. - PubMed
-
- Gheorghiade M, Filippatos G, De Luca L and Burnett J. Congestion in acute heart failure syndromes: an essential target of evaluation and treatment. The American Journal of Medicine. 2006;119:S3–S10. - PubMed
-
- Gheorghiade M, Follath F, Ponikowski P, Barsuk JH, Blair JE, Cleland JG, Dickstein K, Drazner MH, Fonarow GC, Jaarsma T, Jondeau G, Sendon JL, Mebazaa A, Metra M, Nieminen M, Pang PS, Seferovic P, Stevenson LW, van Veldhuisen DJ, Zannad F, Anker SD, Rhodes A, McMurray JJ, Filippatos G, European Society of C and European Society of Intensive Care M. Assessing and grading congestion in acute heart failure: a scientific statement from the acute heart failure committee of the heart failure association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. Eur J Heart Fail. 2010;12:423–33. - PubMed

