New Insights into the Pathogenesis of Diabetic Nephropathy: Proximal Renal Tubules Are Primary Target of Oxidative Stress in Diabetic Kidney
- PMID: 32410750
- PMCID: PMC7212204
- DOI: 10.1267/ahc.20008
New Insights into the Pathogenesis of Diabetic Nephropathy: Proximal Renal Tubules Are Primary Target of Oxidative Stress in Diabetic Kidney
Abstract
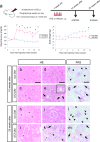
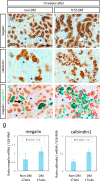
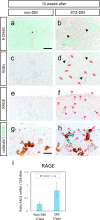
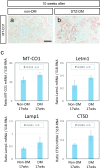
Diabetic nephropathy is a major source of end-stage renal failure, affecting about one-third cases of diabetes mellitus. It has long been accepted that diabetic nephropathy is mainly characterized by glomerular defects, while clinical observations have implied that renal tubular damage is closely linked to kidney dysfunction at the early stages of diabetic nephropathy. In this study, we conducted pathohistological analyses focusing on renal tubular lesions in the early-stage diabetic kidney with the use of a streptozotocin (STZ)-induced diabetes mellitus mouse model. The results revealed that histological alterations in renal tubules, shown by a vacuolar nucleic structure, accumulations of PAS-positive substance, and accelerated restoration stress, occur initially without the presence of glomerular lesions in the early-stage diabetic kidney, and that these tubular defects are localized mainly in proximal renal tubules. Moreover, enhanced expression of RAGE, suggesting an aberrant activation of AGEs-RAGE signaling pathway, and accumulation of oxidative modified mitochondria through the impaired autophagy/lysosome system, were also seen in the damaged diabetic proximal renal tubules. Our findings indicate that proximal tubular defects are the initial pathological events increasingly linked to the progression of diabetic nephropathy, and that controlling renal tubular damage could be an effective therapeutic strategy for the clinical treatment of diabetic nephropathy.
Keywords: AGEs; RAGE; diabetic nephropathy; oxidative stress; proximal renal tubule.
2020 The Japan Society of Histochemistry and Cytochemistry.
Conflict of interest statement
VThe authors declare no conflicts of interest.
Figures

References
-
- Bohlender J. M., Franke S., Stein G. and Wolf G. (2005) Advanced glycation end products and the kidney. Am. J. Physiol. Renal Physiol. 289; F645–659. - PubMed
-
- Brunk U. T. and Terman A. (2002) The mitochondrial-lysosomal axis theory of aging: accumulation of damaged mitochondria as a result of imperfect autophagocytosis. Eur. J. Biochem. 269; 1996–2002. - PubMed

