Azelaic Acid Induces Mitochondrial Biogenesis in Skeletal Muscle by Activation of Olfactory Receptor 544
- PMID: 32411005
- PMCID: PMC7199515
- DOI: 10.3389/fphys.2020.00329
Azelaic Acid Induces Mitochondrial Biogenesis in Skeletal Muscle by Activation of Olfactory Receptor 544
Abstract
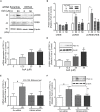
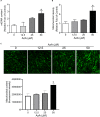
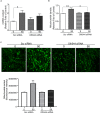
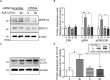
Mouse olfactory receptor 544 (Olfr544) is ectopically expressed in varied extra-nasal organs with tissue specific functions. Here, we investigated the functionality of Olfr544 in skeletal muscle cells and tissue. The expression of Olfr544 is confirmed by RT-PCR and qPCR in skeletal muscle cells and mouse skeletal muscle assessed by RT-PCR and qPCR. Olfr544 activation by its ligand, azelaic acid (AzA, 50 μM), induced mitochondrial biogenesis and autophagy in cultured skeletal myotubes by induction of cyclic adenosine monophosphate-response element binding protein (CREB)-peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α)-extracellular signal-regulated kinase-1/2 (ERK1/2) signaling axis. The silencing Olfr544 gene expression abrogated these effects of AzA in cultured myotubes. Similarly, in mice, the acute subcutaneous injection of AzA induced the CREB-PGC-1α-ERK1/2 pathways in mouse skeletal muscle, but these activations were negated in those of Olfr544 knockout mice. These demonstrate that the induction of mitochondrial biogenesis in skeletal muscle by AzA is Olfr544-dependent. Oral administration of AzA to high-fat-diet fed obese mice for 6 weeks increased mitochondrial DNA content in the skeletal muscle as well. Collectively, these findings demonstrate that Olfr544 activation by AzA regulates mitochondrial biogenesis in skeletal muscle. Intake of AzA or food containing AzA may help to improve skeletal muscle function.
Keywords: azelaic acid; mitochondrial biogenesis; myotube; olfactory receptor 544; skeletal muscle.
Copyright © 2020 Thach, Wu, Hwang and Lee.
Figures

References
-
- Buck L., Axel R. (1991). A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell 65 175–187. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

