More Than Just a Barrier: The Immune Functions of the Airway Epithelium in Asthma Pathogenesis
- PMID: 32411147
- PMCID: PMC7198799
- DOI: 10.3389/fimmu.2020.00761
More Than Just a Barrier: The Immune Functions of the Airway Epithelium in Asthma Pathogenesis
Abstract
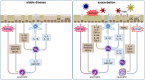
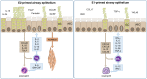
Allergic bronchial asthma is a chronic disease of the airways that is characterized by symptoms like respiratory distress, chest tightness, wheezing, productive cough, and acute episodes of broncho-obstruction. This symptom-complex arises on the basis of chronic allergic inflammation of the airway wall. Consequently, the airway epithelium is central to the pathogenesis of this disease, because its multiple abilities directly have an impact on the inflammatory response and thus the formation of the disease. In turn, its structure and functions are markedly impaired by the inflammation. Hence, the airway epithelium represents a sealed, self-cleaning barrier, that prohibits penetration of inhaled allergens, pathogens, and other noxious agents into the body. This barrier is covered with mucus that further contains antimicrobial peptides and antibodies that are either produced or specifically transported by the airway epithelium in order to trap these particles and to remove them from the body by a process called mucociliary clearance. Once this first line of defense of the lung is overcome, airway epithelial cells are the first cells to get in contact with pathogens, to be damaged or infected. Therefore, these cells release a plethora of chemokines and cytokines that not only induce an acute inflammatory reaction but also have an impact on the alignment of the following immune reaction. In case of asthma, all these functions are impaired by the already existing allergic immune response that per se weakens the barrier integrity and self-cleaning abilities of the airway epithelium making it more vulnerable to penetration of allergens as well as of infection by bacteria and viruses. Recent studies indicate that the history of allergy- and pathogen-derived insults can leave some kind of memory in these cells that can be described as imprinting or trained immunity. Thus, the airway epithelium is in the center of processes that lead to formation, progression and acute exacerbation of asthma.
Keywords: asthma; barrier; imprinting; inflammation; mucus; polarization; trained immunity.
Copyright © 2020 Frey, Lunding, Ehlers, Weckmann, Zissler and Wegmann.
Figures


References
-
- Network GA. The Global Asthma Report 2018. Auckland: Global Asthma Network; (2018).
-
- Gibson GJ, Loddenkemper R, Lundbäck B, Sibille Y. Respiratory health and disease in Europe: the new European Lung white book. Eur Respir J. (2013) 42:559–63. - PubMed
-
- Nurmagambetov T, Kuwahara R, Garbe P. The economic burden of asthma in the United States, 2008-2013. Ann Am Thorac Soc. (2018) 15:348–56. - PubMed
-
- Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. (2006) 355:2226–35. - PubMed

