Towards stem cell therapies for skeletal muscle repair
- PMID: 32411395
- PMCID: PMC7214464
- DOI: 10.1038/s41536-020-0094-3
Towards stem cell therapies for skeletal muscle repair
Abstract
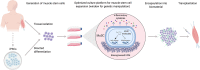
Skeletal muscle is an ideal target for cell therapy. The use of its potent stem cell population in the form of autologous intramuscular transplantation represents a tantalizing strategy to slow the progression of congenital muscle diseases (such as Duchenne Muscular Dystrophy) or regenerate injured tissue following trauma. The syncytial nature of skeletal muscle uniquely permits the engraftment of stem/progenitor cells to contribute to new myonuclei and restore the expression of genes mutated in myopathies. Historically however, the implementation of this approach has been significantly limited by the inability to expand undifferentiated muscle stem cells (MuSCs) in culture whilst maintaining transplantation potential. This is crucial, as MuSC expansion and/or genetic manipulation is likely necessary for therapeutic applications. In this article, we review recent studies that have provided a number of important breakthroughs to tackle this problem. Progress towards this goal has been achieved by exploiting biochemical, biophysical and developmental paradigms to construct innovative in vitro strategies that are guiding stem cell therapies for muscle repair towards the clinic.
Keywords: Cell delivery; Muscle stem cells.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

