Therapeutic vaccination with IDLV-SIV-Gag results in durable viremia control in chronically SHIV-infected macaques
- PMID: 32411399
- PMCID: PMC7210278
- DOI: 10.1038/s41541-020-0186-5
Therapeutic vaccination with IDLV-SIV-Gag results in durable viremia control in chronically SHIV-infected macaques
Abstract
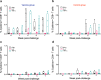
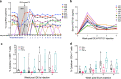
Despite incredible scientific efforts, there is no cure for HIV infection. While antiretroviral treatment (ART) can help control the virus and prevent transmission, it cannot eradicate HIV from viral reservoirs established before the initiation of therapy. Further, HIV-infected individuals reliably exhibit viral rebound when ART is interrupted, suggesting that the host immune response fails to control viral replication in persistent reservoirs. Therapeutic vaccines are one current approach to improving antiviral host immune responses and enhance long term virus control. In the present study, we used an integrase defective lentiviral vector (IDLV) expressing SIV-Gag to boost anti-Gag specific immune responses in macaques chronically infected with the tier-2 SHIV-1157(QNE)Y173H. A single immunization with IDLV-SIV-Gag induced durable (>20 weeks) virus control in 55% of the vaccinated macaques, correlating with an increased magnitude of SIV-Gag specific CD8+ T-cell responses. IDLV-based therapeutic vaccines are therefore an effective approach to improve virus specific CD8+ T-cell responses and mediate virus control.
Keywords: HIV infections; Vaccines.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures



References
Grants and funding
- P01 AI110485/AI/NIAID NIH HHS/United States
- HHSN272201300003I-HHSN27200005/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- HHSN27201100016C/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- 1P01AI110485-01A1/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
LinkOut - more resources
Full Text Sources
Research Materials

