An Erythrocyte Membrane-Associated Antigen, PvTRAg-26 of Plasmodium vivax: A Study of Its Antigenicity and Immunogenicity
- PMID: 32411650
- PMCID: PMC7198802
- DOI: 10.3389/fpubh.2020.00148
An Erythrocyte Membrane-Associated Antigen, PvTRAg-26 of Plasmodium vivax: A Study of Its Antigenicity and Immunogenicity
Abstract
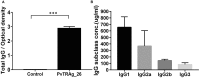
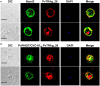
Background:Plasmodium tryptophan-rich (TR) proteins have been proposed as potential vaccine candidate antigens. Among them, P. vivax tryptophan-rich antigens (PvTR-Ags), which have positionally conserved tryptophan residues in a TR domain, are highly antigenic in humans. Several of these antigens, including PvTRAg-26, have exhibited erythrocyte-binding activities. Methods: Subclasses of IgG antibodies against PvTRAg-26 were detected by enzyme-linked immunosorbent assay in 35 P. vivax infected patients and mice immunized with the recombinant antigen to characterize its antigenicity and immunogenicity. Moreover, the antigen-specific immune responses and Th1/Th2-type cytokine patterns of splenocytes from the immunized animals were determined in vitro. The subcellular localization of PvTRAg-26 in ring-stage parasites was also detected by indirect immunofluorescence assay. Results: The IgG1 and IgG3 levels in P. vivax-infected patients were significantly higher than those in uninfected individuals. In the PvTRAg-26-immunized mice, elevated levels of antigen-specific IgG antibodies were observed, dominated by the IgG1 subclass, and Th1-type cytokines were remarkably increased compared with Th2-type cytokines. Additionally, the subcellular location of the PvTRAg-26 protein was closely associated with the caveola-vesicle complex on the infected-erythrocyte membrane in the early ring stage of P. vivax. Conclusions: PvTRAg-26, a P. vivax TR antigen, with high antigenicity and immunogenicity, induces Th1-cytokine response and increases production of IgG1 antibodies. This immune profiling study provided a substantial evidence that PvTRAg-26 may be a potential candidate for P. vivax vaccine development.
Keywords: Plasmodium vivax; immunogenicity; malaria; tryptophan-rich antigens; vaccine candidate.
Copyright © 2020 Fan, Xia, Shen, Fang, Xia, Zheng, Han, Han, Wang and Xu.
Figures



Similar articles
-
Immunoprofiling of the tryptophan-rich antigen family in Plasmodium vivax.Infect Immun. 2015 Aug;83(8):3083-95. doi: 10.1128/IAI.03067-14. Epub 2015 May 18. Infect Immun. 2015. PMID: 25987709 Free PMC article.
-
Plasmodium vivax: immunological properties of tryptophan-rich antigens PvTRAg 35.2 and PvTRAg 80.6.Microbes Infect. 2010 Nov;12(12-13):1019-26. doi: 10.1016/j.micinf.2010.07.004. Epub 2010 Jul 16. Microbes Infect. 2010. PMID: 20638483
-
Cellular immune responses to recombinant Plasmodium vivax tryptophan-rich antigen (PvTRAg) among individuals exposed to vivax malaria.Parasite Immunol. 2008 Jun-Jul;30(6-7):379-83. doi: 10.1111/j.1365-3024.2008.01033.x. Epub 2008 Apr 23. Parasite Immunol. 2008. PMID: 18435687
-
Antigenicity studies in humans and immunogenicity studies in mice: an MSP1P subdomain as a candidate for malaria vaccine development.Microbes Infect. 2014 May;16(5):419-28. doi: 10.1016/j.micinf.2014.02.002. Epub 2014 Feb 20. Microbes Infect. 2014. PMID: 24560875
-
Immunogenicity and antigenicity of a conserved fragment of the rhoptry-associated membrane antigen of Plasmodium vivax.Parasit Vectors. 2022 Nov 15;15(1):428. doi: 10.1186/s13071-022-05561-8. Parasit Vectors. 2022. PMID: 36380374 Free PMC article.
Cited by
-
Dynamics of IgM and IgG Antibody Response Profile against Linear B-Cell Epitopes from Exoerythrocytic (CelTOS and TRAP) and Erythrocytic (CyRPA) Phases of Plasmodium vivax: Follow-Up Study.Antibodies (Basel). 2024 Aug 15;13(3):69. doi: 10.3390/antib13030069. Antibodies (Basel). 2024. PMID: 39189240 Free PMC article.
-
Plasmodium vivax Protein PvTRAg23 Triggers Spleen Fibroblasts for Inflammatory Profile and Reduces Type I Collagen Secretion via NF-κBp65 Pathway.Front Immunol. 2022 Jun 13;13:877122. doi: 10.3389/fimmu.2022.877122. eCollection 2022. Front Immunol. 2022. PMID: 35769479 Free PMC article.
-
The essential genome of Plasmodium knowlesi reveals determinants of antimalarial susceptibility.Science. 2025 Feb 7;387(6734):eadq6241. doi: 10.1126/science.adq6241. Epub 2025 Feb 7. Science. 2025. PMID: 39913579
-
Plasmodium vivax vaccine: What is the best way to go?Front Immunol. 2023 Jan 16;13:910236. doi: 10.3389/fimmu.2022.910236. eCollection 2022. Front Immunol. 2023. PMID: 36726991 Free PMC article. Review.
References
-
- WHO World Malaria Report 2018. (2018).
-
- Mehndiratta S, Rajeshwari K, Dubey AP. Multiple-organ dysfunction in a case of Plasmodium vivax malaria. J Vector Borne Dis. (2013) 50:71-3. - PubMed
-
- Pico de Coana Y, Rodriguez J, Guerrero E, Barrero C, Rodriguez R, Mendoza M, et al. . A highly infective Plasmodium vivax strain adapted to Aotus monkeys: quantitative haematological and molecular determinations useful for P. vivax malaria vaccine development. Vaccine. (2003) 21:3930-7. 10.1016/S0264-410X(03)00278-0 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

