Nicotinamide Mononucleotide: A Promising Molecule for Therapy of Diverse Diseases by Targeting NAD+ Metabolism
- PMID: 32411700
- PMCID: PMC7198709
- DOI: 10.3389/fcell.2020.00246
Nicotinamide Mononucleotide: A Promising Molecule for Therapy of Diverse Diseases by Targeting NAD+ Metabolism
Abstract
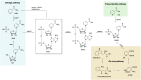
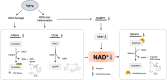
NAD+, a co-enzyme involved in a great deal of biochemical reactions, has been found to be a network node of diverse biological processes. In mammalian cells, NAD+ is synthetized, predominantly through NMN, to replenish the consumption by NADase participating in physiologic processes including DNA repair, metabolism, and cell death. Correspondingly, aberrant NAD+ metabolism is observed in many diseases. In this review, we discuss how the homeostasis of NAD+ is maintained in healthy condition and provide several age-related pathological examples related with NAD+ unbalance. The sirtuins family, whose functions are NAD-dependent, is also reviewed. Administration of NMN surprisingly demonstrated amelioration of the pathological conditions in some age-related disease mouse models. Further clinical trials have been launched to investigate the safety and benefits of NMN. The NAD+ production and consumption pathways including NMN are essential for more precise understanding and therapy of age-related pathological processes such as diabetes, ischemia-reperfusion injury, heart failure, Alzheimer's disease, and retinal degeneration.
Keywords: Alzheimer’s disease; aging; diabetes; nicotinamide adenine dinucleotide (NAD); nicotinamide mononucleotide (NMN); obesity.
Copyright © 2020 Hong, Mo, Zhang, Huang and Wei.
Figures


References
-
- Aredo B., Zhang K., Chen X., Wang C. X., Li T., Ufret-Vincenty R. L. (2015). Differences in the distribution, phenotype and gene expression of subretinal microglia/macrophages in C57BL/6N (Crb1 rd8/rd8) versus C57BL6/J (Crb1 wt/wt) mice. J. Neuroinflammation 12:6. 10.1186/s12974-014-0221-4 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Medical

