Organ System Crosstalk in Cardiometabolic Disease in the Age of Multimorbidity
- PMID: 32411724
- PMCID: PMC7198858
- DOI: 10.3389/fcvm.2020.00064
Organ System Crosstalk in Cardiometabolic Disease in the Age of Multimorbidity
Abstract
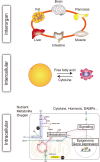
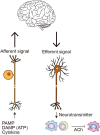
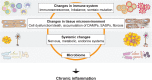
The close association among cardiovascular, metabolic, and kidney diseases suggests a common pathological basis and significant interaction among these diseases. Metabolic syndrome and cardiorenal syndrome are two examples that exemplify the interlinked development of disease or dysfunction in two or more organs. Recent studies have been sorting out the mechanisms responsible for the crosstalk among the organs comprising the cardiovascular, metabolic, and renal systems, including heart-kidney and adipose-liver signaling, among many others. However, it is also becoming clear that this crosstalk is not limited to just pairs of organs, and in addition to organ-organ crosstalk, there are also organ-system and organ-body interactions. For instance, heart failure broadly impacts various organs and systems, including the kidney, liver, lung, and nervous system. Conversely, systemic dysregulation of metabolism, immunity, and nervous system activity greatly affects heart failure development and prognosis. This is particularly noteworthy, as more and more patients present with two or more coexisting chronic diseases or conditions (multimorbidity) due in part to the aging of society. Advances in treatment also contribute to the increase in multimorbidity, as exemplified by cardiovascular disease in cancer survivors. To understand the mechanisms underlying the increasing burden of multimorbidity, it is vital to elucidate the multilevel crosstalk and communication within the body at the levels of organ systems, tissues, and cells. In this article, we focus on chronic inflammation as a key common pathological basis of cardiovascular and metabolic diseases, and discuss emerging mechanisms that drive chronic inflammation in the context of multimorbidity.
Keywords: cardiorenal syndrome; chronic inflammation; clonal hematopoiesis of indeterminate potential (CHIP); heart failure; metabolic syndrome; multimorbidity; organ crosstalk; somatic mutation.
Copyright © 2020 Oishi and Manabe.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources

