DNA Damage, an Innocent Bystander in Atrial Fibrillation and Other Cardiovascular Diseases?
- PMID: 32411727
- PMCID: PMC7198718
- DOI: 10.3389/fcvm.2020.00067
DNA Damage, an Innocent Bystander in Atrial Fibrillation and Other Cardiovascular Diseases?
Abstract
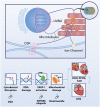
Atrial Fibrillation (AF) is the most common clinical tachyarrhythmia with a strong tendency to progress in time. AF is difficult to treat and therefore there is a great need to dissect root causes of AF with the ultimate goal to develop mechanism-based (drug) therapies. New findings related to mechanisms driving AF progression indicate a prime role for DNA damage-induced metabolic remodeling. A recent study uncovered that AF results in oxidative DNA damage and consequently excessive poly-ADP-ribose polymerase 1 (PARP1) activation and nicotinamide adenine dinucleotide (NAD+) depletion and finally atrial cardiomyocyte electrical and contractile dysfunction. This newly elucidated role of DNA damage in AF opens opportunities for novel therapeutic strategies. Recently developed PARP inhibitors, such as ABT-888 and olaparib, provide beneficial effects in limiting experimental AF, and are also found to limit atherosclerotic coronary artery disease and heart failure. Another therapeutic option to protect against AF is to replenish the NAD+ pool by supplementation with NAD+ or its precursors, such as nicotinamide and nicotinamide riboside. In this review, we describe the role of DNA damage-mediated metabolic remodeling in AF and other cardiovascular diseases, discuss novel druggable targets for AF and highlight future directions for clinical trials with drugs directed at PARP1-NAD+ pathway with the ultimate aim to preserve quality of life and to attenuate severe complications such as heart failure or stroke in patients with AF.
Keywords: DNA damage; PARP; atrial cardiomyopathy; atrial fibrillation; dilated cardiomyopathy; lamin a/c; metabolism; peripartum cardiomyopathy.
Copyright © 2020 Ramos and Brundel.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

