CAR-T Cell Therapy in Cancer: Tribulations and Road Ahead
- PMID: 32411789
- PMCID: PMC7201836
- DOI: 10.1155/2020/1924379
CAR-T Cell Therapy in Cancer: Tribulations and Road Ahead
Abstract
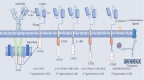
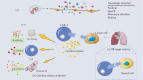
Chimeric antigen receptor- (CAR-) T cell therapy is one of the most recent innovative immunotherapies and is rapidly evolving. Like other technologies, CAR-T cell therapy has undergone a long development process, and persistent explorations of the actions of the intracellular signaling domain and make several improvements have led to the superior efficacy when anti-CD19 CAR-T cell treatments in B cell cancers. At present, CAR-T cell therapy is developing rapidly, and many clinical trials have been established on a global scale, which has great commercial potential. This review mainly describes the toxicity of CAR-T cell therapy and the challenges of CAR-T cells in the treatment of solid tumors, and looks forward to future development and opportunities for immunotherapy and reviews major breakthroughs in CAR-T cell therapy.
Copyright © 2020 Qingyang Zhang et al.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

