Lessons Learned from Experimental Human Model of Zinc Deficiency
- PMID: 32411807
- PMCID: PMC7199614
- DOI: 10.1155/2020/9207279
Lessons Learned from Experimental Human Model of Zinc Deficiency
Abstract
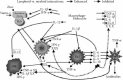
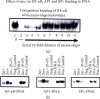
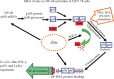
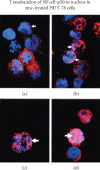
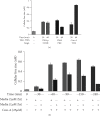
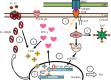
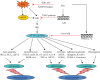
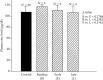
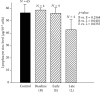
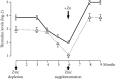
Zinc is an essential element for humans, and its deficiency was documented in 1963. Nutritional zinc deficiency is now known to affect over two billion subjects in the developing world. Conditioned deficiency of zinc in many diseases has also been observed. In zinc-deficient dwarfs from the Middle East, we reported growth retardation, delayed sexual development, susceptibility to infections, poor appetite, and mental lethargy. We never found a zinc-deficient dwarf who survived beyond the age of 25 y. In an experimental model of human mild zinc deficiency, we reported decreased thymulin (a thymopoietic hormone) activity in Th1 cells, decreased mRNAs of IL-2 and IFN-gamma genes, and decreased activity of natural killer cells (NK) and T cytotoxic T cells. The effect of zinc deficiency on thymulin activity and IL-2 mRNA was seen within eight to twelve weeks of the institution of zinc-deficient diet in human volunteers, whereas lymphocyte zinc decreased in 20 weeks and plasma zinc decreased in 24 weeks after instituting zinc-deficient diet. We hypothesized that decreased thymulin activity, which is known to proliferate Th1 cells, decreased the proliferation differentiation of Th1 cells. This resulted in decreased generation of IL-2 and IFN-gamma. We observed no effect in Th2 cell function; thus, zinc deficiency resulted in an imbalance of Th1 to Th2 function resulting in decreased cell-mediated immunity. Zinc therapy may be very useful in many chronic diseases. Zinc supplementation improves cell-mediated immunity, decreases oxidative stress, and decreases generation of chronic inflammatory cytokines in humans. Development of sensitive immunological biomarkers may be more sensitive than an assay of zinc in plasma and peripheral blood cells for diagnosis of marginal zinc deficiency in human.
Copyright © 2020 Ananda S. Prasad.
Conflict of interest statement
The author declares no conflict of interest.
Figures

References
-
- Raulin J. Chemical studies on vegetation. Annals of Science Nature. 1869;XI:93–99.
-
- Todd W. R., Elvehjem C. A., Hart E. B. Zinc in the nutrition of the rat. American Journal of Physiology. 1933;107(1):146–156. doi: 10.1152/ajplegacy.1933.107.1.146. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

