Androgen sensitivity gateway to COVID-19 disease severity
- PMID: 32412125
- PMCID: PMC7273095
- DOI: 10.1002/ddr.21688
Androgen sensitivity gateway to COVID-19 disease severity
Abstract
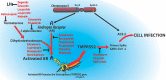
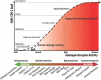
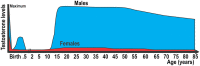
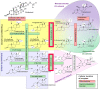
In this communication, we present arguments for androgen sensitivity as a likely determinant of COVID-19 disease severity. The androgen sensitivity model explains why males are more likely to develop severe symptoms while children are ostensibly resistant to infection. Further, the model explains the difference in COVID-19 mortality rates among different ethnicities. Androgen sensitivity is determined by genetic variants of the androgen receptor. The androgen receptor regulates transcription of the transmembrane protease, serine 2 (TMPRSS2), which is required for SARS-CoV-2 infectivity. TMPRSS2 primes the Spike protein of the virus, which has two consequences: diminishing viral recognition by neutralizing antibodies and activating SARS-CoV-2 for virus-cell fusion. Genetic variants that have been associated with androgenetic alopecia, prostate cancer, benign prostatic hyperplasia and polycystic ovary syndrome could be associated with host susceptibility. In addition to theoretical epidemiological and molecular mechanisms, there are reports of high rates of androgenetic alopecia of from hospitalized COVID-19 patients due to severe symptoms. Androgen sensitivity is a likely determinant of COVID-19 disease severity. We believe that the evidence presented in this communication warrants the initiation of trials using anti-androgen agents.
Keywords: COVID-19; SARS-CoV-2; TMPRSS2; alopecia; androgens; anti-androgen; clinical trial; pandemic.
© 2020 Wiley Periodicals, Inc.
Figures
References
-
- Afar, D. E. H. , et al. (2001). Catalytic cleavage of the androgen‐regulated TMPRSS2 protease results in its secretion by prostate and prostate cancer epithelia. Cancer Research, 61, 1686–1692. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11245484 - PubMed
-
- Bennett, C. L. , Price, D. K. , Kim, S. , Liu, D. , Jovanovic, B. D. , Nathan, D. , … Figg, W. D. (2002). Racial variation in CAG repeat lengths within the androgen receptor gene among prostate cancer patients of lower socioeconomic status. Journal of Clinical Oncology, 20, 3599–3604. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

