Clinical characteristics and predictive factors of survival of 761 cancer patients on home parenteral nutrition: A prospective, cohort study
- PMID: 32412178
- PMCID: PMC7333857
- DOI: 10.1002/cam4.3064
Clinical characteristics and predictive factors of survival of 761 cancer patients on home parenteral nutrition: A prospective, cohort study
Abstract
Background: Robust data reporting the survival of cancer patients on home parenteral nutrition (HPN) are lacking. The aim of this prospective, cohort study was to investigate clinical characteristics, predictive factors, and overall survival (OS) of adult-malnourished cancer patients eligible for HPN according to the European guideline recommendations.
Methods: During the study period, 1658 cancer patients were consecutively evaluated in a tertiary university hospital. Of these, 761 who received HPN were grouped into four cohorts according to the provision of supplemental PN (SPN) or total (TPN) and whether they received chemotherapy (CT+ or CT- ): SPN/CT+ (n = 376), TPN/CT+ (n = 99), SPN/CT- (n = 191), and TPN/CT- (n = 95). Patient demographics, nutritional status, cancer-related characteristics, and prognostic scores assessed at HPN start. The primary outcome was OS.
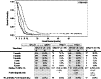
Results: Median OS was 8.9, 4.3, 5.7, and 2.2 months for the SPN/CT+ , TPN/CT+ , SPN/CT- , and TPN/CT- cohorts, respectively. In multivariable analysis, predictors showing significant association with decreased survival were patient cohorts, modified Glasgow Prognostic Score (1 and 2 scores), weight loss (>15%) in the 3 months before HPN start, and TNM IV stage while protective factors of survival were Karnofsky Performance Status (>50), albumin level (>3.5 g/dL), oral protein intake, BMI (>20.5), and weight at HPN start.
Conclusion: For the first time, in four different cohorts of cancer patients on HPN, clinical characteristics and survival were compared. This large study showed that survival is significantly correlated with patient characteristics at HPN start and that the presence of favorable factors may determine even a fourfold increase in survival. These data are expected to assist physicians in the appropriate prescription of HPN.
Keywords: cancer survival; cohort study; home care; medical nutrition therapy; oncologic treatment; supportive care.
© 2020 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
PC reported honoraria for speaking and teaching from Baxter. The other authors declare that they have no competing interests.
Figures
References
-
- Bozzetti F. Nutritional support of the oncology patient. Crit Rev Oncol Hematol. 2013;87(2):172‐200. - PubMed
-
- Arends J, Bachmann P, Baracos V, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36(1):11‐48. - PubMed
-
- August DA, Huhmann MB. American Society for Parenteral and Enteral Nutrition (ASPEN) board of directors. ASPEN clinical guidelines: nutrition support therapy during adult anticancer treatment and in hematopoietic cell transplantation. JPEN J Parenter Enter Nutr. 2009;33(5):472‐500. - PubMed
-
- Arends J, Bodoky G, Bozzetti F, et al. ESPEN guidelines on enteral nutrition: non‐surgical oncology. Clin Nutr. 2006;25(2):245‐259. - PubMed
-
- Bozzetti F, Arends J, Lundholm K, et al. ESPEN Guidelines on Parenteral Nutrition: non‐surgical oncology. Clin Nutr. 2009;28(4):445‐454. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

