Rifapentine Population Pharmacokinetics and Dosing Recommendations for Latent Tuberculosis Infection
- PMID: 32412342
- PMCID: PMC7491398
- DOI: 10.1164/rccm.201912-2489OC
Rifapentine Population Pharmacokinetics and Dosing Recommendations for Latent Tuberculosis Infection
Abstract
Rationale: Rifapentine has been investigated at various doses, frequencies, and dosing algorithms, but clarity on the optimal dosing approach is lacking.Objectives: To characterize rifapentine population pharmacokinetics, including autoinduction, and determine optimal dosing strategies for short-course rifapentine-based regimens for latent tuberculosis infection.Methods: Rifapentine pharmacokinetic studies were identified though a systematic review of literature. Individual plasma concentrations were pooled, and nonlinear mixed-effects modeling was performed. A subset of data was reserved for external validation. Simulations were performed under various dosing conditions, including current weight-based methods; and alternative methods driven by identified covariates.Measurements and Main Results: We identified nine clinical studies with a total of 863 participants with pharmacokinetic data (n = 4,301 plasma samples). Rifapentine population pharmacokinetics were described successfully with a one-compartment distribution model. Autoinduction of clearance was driven by rifapentine plasma concentrations. The maximum effect was a 72% increase in clearance and was reached after 21 days. Drug bioavailability decreased by 27% with HIV infection, decreased by 28% with fasting, and increased by 49% with a high-fat meal. Body weight was not a clinically relevant predictor of clearance. Pharmacokinetic simulations showed that current weight-based dosing leads to lower exposures in individuals with low weight, which can be overcome with flat dosing. In HIV-positive patients, 30% higher doses are required to match drug exposure in HIV-negative patients.Conclusions: Weight-based dosing of rifapentine should be removed from clinical guidelines, and higher doses for HIV-positive patients should be considered to provide equivalent efficacy.
Keywords: latent tuberculosis; population pharmacokinetics; rifamycins; rifapentine; tuberculosis.
Figures
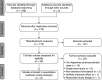
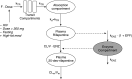

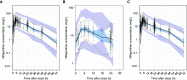
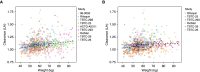
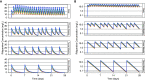
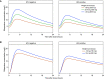
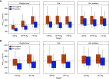

Comment in
-
Simplifying Rifapentine Dosing for Tuberculosis Treatment and Prevention.Am J Respir Crit Care Med. 2020 Sep 15;202(6):793-794. doi: 10.1164/rccm.202006-2144ED. Am J Respir Crit Care Med. 2020. PMID: 32634022 Free PMC article. No abstract available.
References
-
- World Health Organization. Global tuberculosis report 2018. Geneva, Switzerland: WHO Press; 2018.
-
- American Thoracic Society; Centers for Disease Control and Prevention. Targeted tuberculin testing and treatment of latent tuberculosis infection. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. This is a Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC). This statement was endorsed by the Council of the Infectious Diseases Society of America. (IDSA), September 1999, and the sections of this statement as it relates to infants and children were endorsed by the American Academy of Pediatrics (AAP), August 1999. Am J Respir Crit Care Med. 2000;161:S221–S247. - PubMed
-
- Horsburgh CR, Jr, Goldberg S, Bethel J, Chen S, Colson PW, Hirsch-Moverman Y, et al. Tuberculosis Epidemiologic Studies Consortium. Latent TB infection treatment acceptance and completion in the United States and Canada. Chest. 2010;137:401–409. - PubMed
-
- Sterling TR, Villarino ME, Borisov AS, Shang N, Gordin F, Bliven-Sizemore E, et al. TB Trials Consortium PREVENT TB Study Team. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med. 2011;365:2155–2166. - PubMed

