Potency Increase of Spiroketal Analogs of Membrane Inserting Indolyl Mannich Base Antimycobacterials Is Due to Acquisition of MmpL3 Inhibition
- PMID: 32413266
- PMCID: PMC7875313
- DOI: 10.1021/acsinfecdis.0c00121
Potency Increase of Spiroketal Analogs of Membrane Inserting Indolyl Mannich Base Antimycobacterials Is Due to Acquisition of MmpL3 Inhibition
Abstract
Chemistry campaigns identified amphiphilic indolyl Mannich bases as novel membrane-permeabilizing antimycobacterials. Spiroketal analogs of this series showed increased potency, and the lead compound 1 displayed efficacy in a mouse model of tuberculosis. Yet the mechanism by which the spiroketal moiety accomplished the potency "jump" remained unknown. Consistent with its membrane-permeabilizing mechanism, no resistant mutants could be isolated against indolyl Mannich base 2 lacking the spiroketal moiety. In contrast, mutations resistant against spiroketal analog 1 were obtained in mycobacterial membrane protein large 3 (MmpL3), a proton motive force (PMF)-dependent mycolate transporter. Thus, we hypothesized that the potency jump observed for 1 may be due to MmpL3 inhibition acquired by the addition of the spiroketal moiety. Here we showed that 1 inhibited MmpL3 flippase activity without loss of the PMF, colocalized with MmpL3tb-GFP in intact organisms, and yielded a consistent docking pose within the "common inhibitor binding pocket" of MmpL3. The presence of the spiroketal motif in 1 ostensibly augmented its interaction with MmpL3, an outcome not observed in the nonspiroketal analog 2, which displayed no cross-resistance to mmpL3 mutants, dissipated the PMF, and docked poorly in the MmpL3 binding pocket. Surprisingly, 2 inhibited MmpL3 flippase activity, which may be an epiphenomenon arising from its wider membrane disruptive effects. Hence, we conclude that the potency increase associated with the spiroketal analog 1 is linked to the acquisition of a second mechanism, MmpL3 inhibition. In contrast, the nonspiroketal analog 2 acts pleiotropically, affecting several cell membrane-embedded targets, including MmpL3, through its membrane permeabilizing and depolarizing effects.
Keywords: MmpL3; Mycobacterium tuberculosis; cationic amphiphiles; cell membrane; indolyl Mannich bases; membrane permeabilization.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


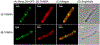



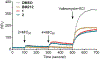
References
-
- The top 10 causes of death, https://www.who.int/news-room/fact-sheets/detail/thetop-10-causes-of-death (accessed March 11, 2020).
-
- World Health Organization (2019). Global tuberculosis report 2019. WHO Press, Geneva, Switzerland.
-
- FDA Approves New Treatment for Highly Drug-Resistant Forms of Tuberculosis, https://www.tballiance.org/news/fda-approves-new-treatment-highly-drug-r... (accessed March 11, 2020).
-
- Yang T, Moreira W, Nyantakyi SA, Chen H, Aziz DB, Go M-L, Dick T (2017) Amphiphilic indole derivatives as antimycobacterial agents: structure-activity relationships and membrane targeting properties. J. Med. Chem 60, 2745–2763. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

