The Median Eminence, A New Oligodendrogenic Niche in the Adult Mouse Brain
- PMID: 32413277
- PMCID: PMC7355143
- DOI: 10.1016/j.stemcr.2020.04.005
The Median Eminence, A New Oligodendrogenic Niche in the Adult Mouse Brain
Abstract
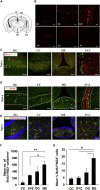
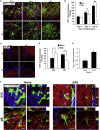
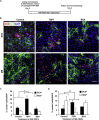
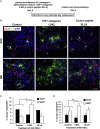
The subventricular zone (SVZ) of the lateral ventricles and the subgranular zone (SGZ) of the dentate gyrus in the hippocampus are known as neurogenic niches. We show that the median eminence (ME) of the hypothalamus comprises BrdU+ newly proliferating cells co-expressing NG2 (oligodendrocyte progenitors) and RIP (pre-myelinating oligodendrocytes), suggesting their differentiation toward mature oligodendrocytes (OLs). ME cells can generate neurospheres (NS) in vitro, which differentiate mostly to OLs compared with SVZ-NS that typically generate neurons. Interestingly, this population of oligodendrocyte progenitors is increased in the ME from experimental autoimmune encephalomyelitis (EAE)-affected mice. Notably, the thrombospondin 1 (TSP1) expressed by astrocytes, acts as negative regulator of oligodendrogenesis in vitro and is downregulated in the ME of EAE mice. Importantly, transplanted ME-NS preferentially differentiate to MBP+ OLs compared with SVZ-NS in Shiverer mice. Hence, discovering the ME as a new site for myelin-producing cells has a great importance for advising future therapy for demyelinating diseases and spinal cord injury.
Keywords: corpus callosum; experimental autoimmune encephalomyelitis; median eminence; multiple sclerosis; myelin repair; neural stem cells; oligodendrocyte progenitors cells; subventricular zone.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Altman J., Das G.D. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 1965;124:319–335. - PubMed
-
- Alvarez-Buylla A., Seri B., Doetsch F. Identification of neural stem cells in the adult vertebrate brain. Brain Res. Bull. 2002;57:751–758. - PubMed
-
- Armada-Moreira A., Ribeiro F.F., Sebastião A.M., Xapelli S. Neuroinflammatory modulators of oligodendrogenesis. Neuroimmunol. Neuroinflam. 2015;2:263–273.
-
- Bedard A., Cossette M., Levesque M., Parent A. Proliferating cells can differentiate into neurons in the striatum of normal adult monkey. Neurosci. Lett. 2002;328:213–216. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

