Material-driven fibronectin assembly rescues matrix defects due to mutations in collagen IV in fibroblasts
- PMID: 32413593
- PMCID: PMC8822498
- DOI: 10.1016/j.biomaterials.2020.120090
Material-driven fibronectin assembly rescues matrix defects due to mutations in collagen IV in fibroblasts
Abstract
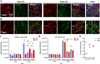
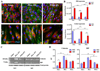
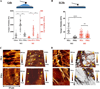
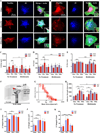
Basement membranes (BMs) are specialised extracellular matrices that provide structural support to tissues as well as influence cell behaviour and signalling. Mutations in COL4A1/COL4A2, a major BM component, cause a familial form of eye, kidney and cerebrovascular disease, including stroke, while common variants in these genes are a risk factor for intracerebral haemorrhage in the general population. These phenotypes are associated with matrix defects, due to mutant protein incorporation in the BM and/or its absence by endoplasmic reticulum (ER) retention. However, the effects of these mutations on matrix stiffness, the contribution of the matrix to the disease mechanism(s) and its effects on the biology of cells harbouring a collagen IV mutation remain poorly understood. To shed light on this, we employed synthetic polymer biointerfaces, poly(ethyl acrylate) (PEA) and poly(methyl acrylate) (PMA) coated with ECM proteins laminin or fibronectin (FN), to generate controlled microenvironments and investigate their effects on the cellular phenotype of primary fibroblasts harbouring a COL4A2+/G702D mutation. FN nanonetworks assembled on PEA induced increased deposition and assembly of collagen IV in COL4A2+/G702D cells, which was associated with reduced ER size and enhanced levels of protein chaperones such as BIP, suggesting increased protein folding capacity of the cell. FN nanonetworks on PEA also partially rescued the reduced stiffness of the deposited matrix and cells, and enhanced cell adhesion through increased actin-myosin contractility, effectively rescuing some of the cellular phenotypes associated with COL4A1/4A2 mutations. The mechanism by which FN nanonetworks enhanced the cell phenotype involved integrin β1-mediated signalling. Collectively, these results suggest that biomaterials and enhanced integrin signalling via assembled FN are able to shape the matrix and cellular phenotype of the COL4A2+/G702D mutation in patient-derived cells.
Keywords: Cell adhesion; Cerebrovascular disease; Collagen IV; Disease mechanism; Extracellular matrix; Protein folding.
Copyright © 2020 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- LeBleu VS, Macdonald B, Kalluri R. Structure and function of basement membranes. Experimental biology and medicine (Maywood, NJ). 2007;232:1121–9. - PubMed
-
- Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nature reviews Cancer. 2003;3:422–33. - PubMed
-
- Van Agtmael T, Bruckner-Tuderman L. Basement membranes and human disease. Cell and tissue research. 2010;339:167–88. - PubMed
-
- Timpl R, Aumailley M. Biochemistry of basement membranes. Advances in nephrology from the Necker Hospital. 1989;18:59–76. - PubMed

