Mitochondrial Dysfunction: A Common Hallmark Underlying Comorbidity between sIBM and Other Degenerative and Age-Related Diseases
- PMID: 32413985
- PMCID: PMC7290779
- DOI: 10.3390/jcm9051446
Mitochondrial Dysfunction: A Common Hallmark Underlying Comorbidity between sIBM and Other Degenerative and Age-Related Diseases
Abstract
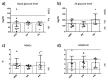
Sporadic inclusion body myositis (sIBM) is an inflammatory myopathy associated, among others, with mitochondrial dysfunction. Similar molecular features are found in Alzheimer's disease (AD) and Type 2 Diabetes Mellitus (T2DM), underlying potential comorbidity. This study aims to evaluate common clinical and molecular hallmarks among sIBM, AD, and T2DM. Comorbidity with AD was assessed in n = 14 sIBM patients by performing neuropsychological and cognitive tests, cranial magnetic resonance imaging, AD cerebrospinal fluid biomarkers (levels of amyloid beta, total tau, and phosphorylated tau at threonine-181), and genetic apolipoprotein E genotyping. In the same sIBM cohort, comorbidity with T2DM was assessed by collecting anthropometric measures and performing an oral glucose tolerance test and insulin determinations. Results were compared to the standard population and other myositis (n = 7 dermatomyositis and n = 7 polymyositis). Mitochondrial contribution into disease was tested by measurement of oxidative/anaerobic and oxidant/antioxidant balances, respiration fluxes, and enzymatic activities in sIBM fibroblasts subjected to different glucose levels. Comorbidity of sIBM with AD was not detected. Clinically, sIBM patients showed signs of misbalanced glucose homeostasis, similar to other myositis. Such misbalance was further confirmed at the molecular level by the metabolic inability of sIBM fibroblasts to adapt to different glucose conditions. Under the standard condition, sIBM fibroblasts showed decreased respiration (0.71 ± 0.08 vs. 1.06 ± 0.04 nmols O2/min; p = 0.024) and increased anaerobic metabolism (5.76 ± 0.52 vs. 3.79 ± 0.35 mM lactate; p = 0.052). Moreover, when glucose conditions were changed, sIBM fibroblasts presented decreased fold change in mitochondrial enzymatic activities (-12.13 ± 21.86 vs. 199.22 ± 62.52 cytochrome c oxidase/citrate synthase ratio; p = 0.017) and increased oxidative stress per mitochondrial activity (203.76 ± 82.77 vs. -69.55 ± 21.00; p = 0.047), underlying scarce metabolic plasticity. These findings do not demonstrate higher prevalence of AD in sIBM patients, but evidences of prediabetogenic conditions were found. Glucose deregulation in myositis suggests the contribution of lifestyle conditions, such as restricted mobility. Additionally, molecular evidences from sIBM fibroblasts confirm that mitochondrial dysfunction may play a role. Monitoring T2DM development and mitochondrial contribution to disease in myositis patients could set a path for novel therapeutic options.
Keywords: Alzheimer 2; T2DM 3; comorbidity 5; mitochondria 4; myositis 6; sIBM 1.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Badrising U.A., Maat-Schieman M., Van Duinen S.G., Breedveld F., Van Doorn P., Van Engelen B., Van Den Hoogen F., Hoogendijk J., Höweler C., De Jager A., et al. Epidemiology of inclusion body myositis in the Netherlands: A nationwide study. Neurology. 2000;55:1385–1387. doi: 10.1212/WNL.55.9.1385. - DOI - PubMed
-
- Tan J.A., Roberts-Thomson P.J., Blumbergs P., Hakendorf P., Cox S.R., Limaye V. Incidence and prevalence of idiopathic inflammatory myopathies in South Australia: A 30-year epidemiologic study of histology-proven cases. Int. J. Rheum. Dis. 2013;16:331–338. doi: 10.1111/j.1756-185X.2011.01669.x. - DOI - PubMed

