Palmitic Acid Affects Intestinal Epithelial Barrier Integrity and Permeability In Vitro
- PMID: 32414055
- PMCID: PMC7278681
- DOI: 10.3390/antiox9050417
Palmitic Acid Affects Intestinal Epithelial Barrier Integrity and Permeability In Vitro
Abstract
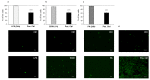
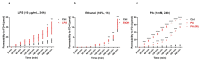
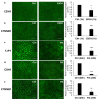
Palmitic acid (PA), a long-chain saturated fatty acid, might activate innate immune cells. PA plays a role in chronic liver disease, diabetes and Crohn's disease, all of which are associated with impaired intestinal permeability. We investigated the effect of PA, at physiological postprandial intestinal concentrations, on gut epithelium as compared to lipopolysaccharide (LPS) and ethanol, using an in vitro gut model, the human intestinal epithelial cell line Caco-2 grown on transwell inserts. Cytotoxicity and oxidative stress were evaluated; epithelial barrier integrity was investigated by measuring the paracellular flux of fluorescein, and through RT-qPCR and immunofluorescence of tight junction (TJ) and adherens junction (AJ) mRNAs and proteins, respectively. In PA-exposed Caco-2 monolayers, cytotoxicity and oxidative stress were not detected. A significant increase in fluorescein flux was observed in PA-treated monolayers, after 90 min and up to 360 min, whereas with LPS and ethanol, this was only observed at later time-points. Gene expression and immunofluorescence analysis showed TJ and AJ alterations only in PA-exposed monolayers. In conclusion, PA affected intestinal permeability without inducing cytotoxicity or oxidative stress. This effect seemed to be faster and stronger than those with LPS and ethanol. Thus, we hypothesized that PA, besides having an immunomodulatory effect, might play a role in inflammatory and functional intestinal disorders in which the intestinal permeability is altered.
Keywords: Caco-2 monolayer; adherens junctions.; gut barrier integrity; intestinal permeability; palmitic acid; tight junctions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Guarino M.P.L., Sessa R., Altomare A., Cocca S., Di Pietro M., Carotti S., Schiavoni G., Alloni R., Emerenziani S., Morini S., et al. Human colonic myogenic dysfunction induced by mucosal lipopolysaccharide translocation and oxidative stress. Dig. Liver Dis. 2013;45:1011–1016. doi: 10.1016/j.dld.2013.06.001. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

