Bioresorbable Polymeric Scaffold in Cardiovascular Applications
- PMID: 32414114
- PMCID: PMC7279389
- DOI: 10.3390/ijms21103444
Bioresorbable Polymeric Scaffold in Cardiovascular Applications
Abstract
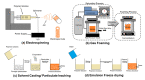
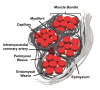
Advances in material science and innovative medical technologies have allowed the development of less invasive interventional procedures for deploying implant devices, including scaffolds for cardiac tissue engineering. Biodegradable materials (e.g., resorbable polymers) are employed in devices that are only needed for a transient period. In the case of coronary stents, the device is only required for 6-8 months before positive remodelling takes place. Hence, biodegradable polymeric stents have been considered to promote this positive remodelling and eliminate the issue of permanent caging of the vessel. In tissue engineering, the role of the scaffold is to support favourable cell-scaffold interaction to stimulate formation of functional tissue. The ideal outcome is for the cells to produce their own extracellular matrix over time and eventually replace the implanted scaffold or tissue engineered construct. Synthetic biodegradable polymers are the favoured candidates as scaffolds, because their degradation rates can be manipulated over a broad time scale, and they may be functionalised easily. This review presents an overview of coronary heart disease, the limitations of current interventions and how biomaterials can be used to potentially circumvent these shortcomings in bioresorbable stents, vascular grafts and cardiac patches. The material specifications, type of polymers used, current progress and future challenges for each application will be discussed in this manuscript.
Keywords: biomaterials; bioresorbable scaffolds; cardiac patches; cardiovascular tissue engineering; polymeric scaffolds; vascular grafts.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- WHO Cardiovascular Diseases (CVDs) [(accessed on 9 March 2020)]; Available online: https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-disea...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

