Improving Anti-Neurodegenerative Benefits of Acetylcholinesterase Inhibitors in Alzheimer's Disease: Are Irreversible Inhibitors the Future?
- PMID: 32414155
- PMCID: PMC7279429
- DOI: 10.3390/ijms21103438
Improving Anti-Neurodegenerative Benefits of Acetylcholinesterase Inhibitors in Alzheimer's Disease: Are Irreversible Inhibitors the Future?
Abstract
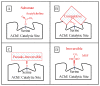
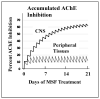
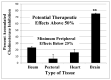
Decades of research have produced no effective method to prevent, delay the onset, or slow the progression of Alzheimer's disease (AD). In contrast to these failures, acetylcholinesterase (AChE, EC 3.1.1.7) inhibitors slow the clinical progression of the disease and randomized, placebo-controlled trials in prodromal and mild to moderate AD patients have shown AChE inhibitor anti-neurodegenerative benefits in the cortex, hippocampus, and basal forebrain. CNS neurodegeneration and atrophy are now recognized as biomarkers of AD according to the National Institute on Aging-Alzheimer's Association (NIA-AA) criteria and recent evidence shows that these markers are among the earliest signs of prodromal AD, before the appearance of amyloid. The current AChE inhibitors (donepezil, rivastigmine, and galantamine) have short-acting mechanisms of action that result in dose-limiting toxicity and inadequate efficacy. Irreversible AChE inhibitors, with a long-acting mechanism of action, are inherently CNS selective and can more than double CNS AChE inhibition possible with short-acting inhibitors. Irreversible AChE inhibitors open the door to high-level CNS AChE inhibition and improved anti-neurodegenerative benefits that may be an important part of future treatments to more effectively prevent, delay the onset, or slow the progression of AD.
Keywords: Alzheimer’s disease; acetylcholinesterase; acetylcholinesterase inhibitor; atrophy; butyrylcholinesterase; donepezil; galantamine; methanesulfonyl fluoride; metrifonate; rivastigmine.
Conflict of interest statement
DEM is Co-Manager of Brain Tools, LLC (Oregon).
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

